PHOTOSENSITIZERS in MEDICINE
Kristian Berg
Department of Radiation Biology
The Norwegian Radium Hospital
Montebello, N-0310 Norway
Kristian.Berg@rr-research.no
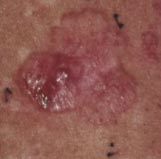
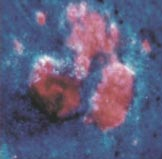
The picture below (left) shows a basal cell carcinoma. A physician wants to treat this skin cancer, but is unsure about the extent of the malignant tissue. What part of the lesion is cancerous? Where are the borders? Physicians can use photosensitizers to answer these questions. They can treat the area with a photosensitizer, such as 5-aminolevulinic acid, and then look at it using a blue light, which causes the photosensitizer to fluoresce (right). The pink/red fluorescent areas define the extent of the malignant lesion. In this module you will learn more about photosensitizers and the properties that make them useful for applications like this.

Photosensitizers have evolved over millions of years, mainly for defence against microbial and herbivorous attack. Some of these compounds have been found to have therapeutic properties, and these and derivatives of these compounds as well as new chemical entities, have been developed for therapeutic and diagnostic purposes. Other biomolecules have the potential to become photosensitizers upon minor modifications, and some man-made compounds have photosensitizer properties that are mostly unwanted. Here, some basic properties of photosentizers will be described, followed by an overview of the most important groups of known photosensitizers.
Review of Basic Properties of Photosentizers
The first law of photochemistry states that "only light absorbed by a molecule can produce photochemical change in that molecule". When light encounters a molecule, it can either be scattered or absorbed. A molecule that has absorbed a quantum of light is said to be excited, and molecules that can be excited by the absorption of visible light are named chromophores . The absorption spectrum of a molecule reflects its probability of transition between the ground state and any of the vibrational and rotational levels of its first and higher excited states. The absorption spectrum, characterized by the molar absorption coefficient (previously known as the extinction coefficient) at a particular wavelength, is described by Beer-Lambert's law:
where
A molecule that has absorbed a light quantum is excited to the first or higher excited states (see the modules on Basic Photophysics and Photochemistry). The higher excited states are dissipated very fast (ps) down to the first excited singlet state with a lifetime of the order of ns. This excited state can be deactivated by the release of heat (nonradiative decay), emitted as fluorescence of a wavelength equal to or longer than that of the excitation light, or by undergoing intersystem crossing (ISC). Photochemistry may also occur, but usually with a low probability, due to the short lifetime of this state.
Exceptions are intramolecular modifications, e.g., those that cause photoisomerization, as in bilirubin, upon the light treatment of hyperbilirubinemia (jaundice) in newborns. ISC is usually forbidden (selection rules), but macrocyclic molecules with conjugated double bond systems (
The Fluorescence of Photosensitizers
Most photosensitizers emit some of the energy from the first excited singlet state as fluorescence. Since the emitted light is usually less energetic than the absorbed light, the emitted light is usually of higher wavelength. The energy of light is inversely proportional to the wavelength.
Two different spectra are usually measured when the fluorescence properties of photosensitizers are evaluated, the fluorescence excitation and emission spectra (Figure 1). The fluorescence excitation spectra are obtained when the emission is detected at one wavelength, and the excitation wavelength is varied. For photosensitizers that are in their monomeric form (not aggregated), the fluorescence excitation spectra and absorption spectra are identical in shape. Aggregated photosensitizers usually show low or no fluorescence. A fluorescence emission spectrum is obtained when the excitation wavelength is kept constant, and the wavelength for the detection of fluorescence is varied. The difference between the excitation and emission peak is called the Stoke's shift.
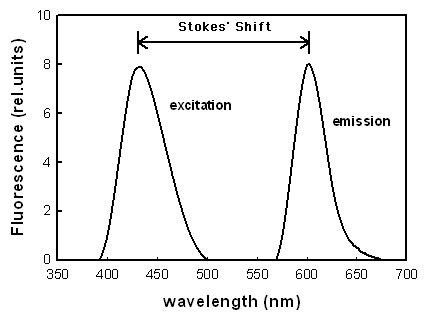
Figure 1. Schematic illustration of fluorescence excitation and emission spectra of dyes.
The fluorescence properties of photosensitizers may be utilized for the detection of cancers. Many photosensitizers preferentially accumulate in neoplastic tissues, and their fluorescence properties may be beneficial in the detection and diagnosis of such lesions (Figure 2). Intravesical instillation of 5-aminolevulinic acid(ALA)-hexyl ester (Hexvix®) (resulting in protoporphyrin IX(PpIX)-based fluorescence) is approved for the detection of bladder cancer, in particular carcinoma in situ, which is difficult to detect. The fluorescence guided resection of bladder cancer has been found promising for treatment purposes. Similarly, systemic 5-ALA (Gliolan) has been approved for intra-operative fluorescence guided detection of residual glioma, and is used for the further resection of remaining tumor tissue.
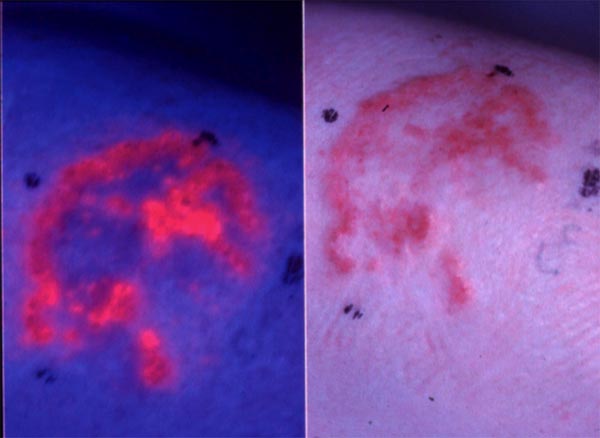
Figure 2. Fluorescence emitted from basal carcinomas treated with 5-aminolevulinic acid methyl ester (5-ALA ME). A basal cell carcinoma is shown in the right figure. The same lesion is treated with 5-ALA ME and 3 hrs later exposed to blue light. Red fluorescence from protoporphyrin IX induced by 5-ALA ME in the lesion is shown on the left side.
Efficacy of Photosensitizers in Producing Chemical Changes in Target Molecules
A photosensitizer is defined as a chemical entity, which upon absorption of light induces a chemical and physical alteration of another chemical entity.
A good photosensitizer should absorb photons efficiently (i.e., high absorption coefficient), have a high quantum yield of triplet formation, and the triplet state should be long lived in order to have time to react with neighbouring target molecules. Most compounds that form triplet states that are able to produce radicals and reactive oxygen species have a tricyclic, heterocyclic or porphyrin-like ring structures with conjugated double bonds (
Similarly, oxygen may be replaced with sulphur or selenium, and the quantum yield of singlet oxygen formation is dramatically increased, such as in merocyanine 540 (MC540) and Nile blue. The environment of the sensitizer may also influence the photosensitising properties of the compound. Solvents that lead to aggregation of the photosensitizer reduce the probabililty of the compound to undergo intersystem crossing, and most of the energy in the excited state will be dissipated through nonradiative decay (internal convertion), producing only heat. Even dimers of the photosensitizer can be photochemically inactive. The absorption spectrum of aggregated photosensitizers is usually different from that of the monomeric form. Binding to proteins or other macromolecules often leads to a redshift in the absorption spectrum, increased lifetime of the triplet state, and deaggregation of aggregated species.
Quantum Yield of Photoinactivation
Einstein introduced the concept of quantum yield,
(
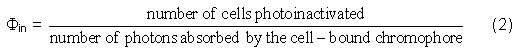
Usually values for
where OD (
Action Spectra
An action spectrum is a presentation of a measured effect as a function of the wavelength of excitation. Such spectra can be used to evaluate the effect of a photosensitizer on producing an event such as cell killing. Action spectra can be used to document the identity of the photosensitizer, since the action spectrum will match the absorption spectrum of the active form of a photosensitizer. The action spectrum for the photochemical inactivation of cells can be found by measuring the dose of light necessary for inactivating the same fraction of the cells at different wavelength, and plotting the results as the dose required vs. wavelength of excitation light.
Porphyrin-Based Photosensitizers
In the following, the photosensitizers are grouped on the basis of their chemical structures, and some of their photosensitizing properties will be described. In addition some diseases caused by photosensitizers, as well as drugs inducing skin photosensitivity will be presented. Specialized photoreceptors such as retinoids and chlorophyll, as well as non-specialized photoacceptors that may have biostimulatory effects upon exposure to visible (400-750 nm) light will be described elsewhere (see modules under Non-Visual Photoreceptors, Vision and Photomorphogenesis). Photosensitizers that are excited by UV light, and are constituents of most normal cells (e.g., riboflavin and flavin mononucleotide), will not be covered here. Hundreds of different compounds have been shown to be photosensitizers for biological systems, and only some selected groups will be presented here.
Porphyrins comprise of four pyrrole subunits linked together by four methine bridges (Figure 3). This tetrapyrrole ring structure is named porphin, and derivatives of porphins are named porphyrins. Tetrapyrroles are naturally occurring pigments, which are used in many biological processes and include the metallopigments heme (the prosthetic group of proteins like hemoglobin, cytochromes, catalase, peroxidase and tryptophane pyrrolase), vitamin B12 , chlorophyll, siroheme (in nitrite and sulphite reductases) and factor F430 (cofactor of methyl CoM reductase). All these compounds are synthesized with uroporphyrinogen III as a common intermediate, and modified to permit coordination of different metals at the ring centre, i.e., Fe in heme and siroheme, Mg in chlorophyll, Co in Vitamin B12, and Ni in factor F430 . These tetrapyrroles do not induce any photochemical or photobiophysical reactions in other compounds or are rapidly quenched in their normal surroundings, e.g., chlorophyll.
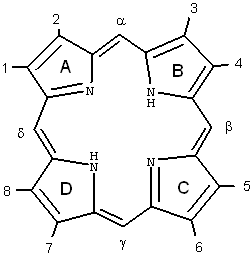
Figure 3. Structure and nomenclature of tetrapyrroles.
By removing the metal, tetrapyrroles become efficient photosensitizers, e.g.,
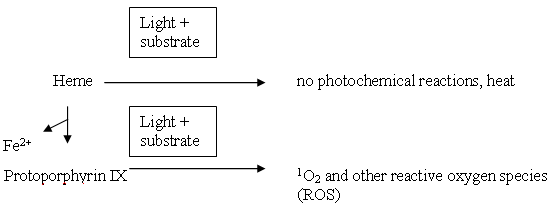
The presence of a coordinated metal ion and its electronic properties are of importance for the photocytotoxic potential of porphyrins as photosensitisers. Most efficient porphyrin-based photosensitizers generally lack coordinated metal ions. Coordinated metal ions increase the probability for the nonradiative decay of the triplet state; paramagnetic metals like Fe3+ being much more efficient than diamagnetic metals like Al3+ and Mg2+. Several metallophotosensitizers have been developed for clinical purposes. Although in most cases, they have lower quantum yields for cell inactivation than they would have in the absence of metal ions, they have other properties like improved solubility and stability, which makes them interesting as therapeutic substances. The metals used include Zn, Pd, Sn, Ru, Pt and Al.
Porphyrin-Based Photosensitizers Used in Photodynamic Therapy (PDT)
PDT is a form of photochemotherapy that requires oxygen for its therapeutic effect, i.e., in PDT a photosensitizer, light and oxygen are all required. The Type II reaction pathway is expected to be the main pathway, but Type I reactions involving oxygen may also be involved (see Basic Photochemistry). PDT has been developed for cancer treatment, precancereous lesions (actinic keratosis) and age-related macular degeneration. Most photosensitizers, and all clinically-approved photosensitizers used in PDT, with the exception of methylene blue, are based on the tetrapyrrole macrocycle. PDT is described more completely in the Photomedicine section, and only some properties related to the photosensitizers will be described here.
Porphyrins and porphyrin-related dyes used in PDT have substituents in the peripheral positions of the pyrrole rings (1-8), on the four methine carbons (meso-positions), and/or coordinated metals. These derivates are synthesized to influence the water/lipid solubility, amphiphilicity, pKa and stability of the compounds. These parameters determine the biodistribution of the compounds, i.e., the intracellular localization, tissue distribution and pharmacokinetics.
Hematoporphyrin (Figure 4) was the first porphyrin to be used on humans in that Dr. Meyer-Betz injected 200 mg of this compound into himself, and exposed his forearm to light from a Finsen lamp, and his hands and face to sunlight. The exposed areas showed clear signs of the photodynamic effect, such as erythema and oedema.
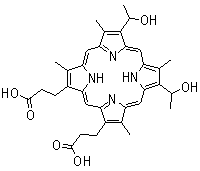
Figure 4. Hematoporhyrin IX.
In the early 1960s, Lipson and colleagues, based upon work by Samuel Schwartz, tried to purify hematoporhyrin by treating it with sulphuric acid in acetic acid followed by treatment with alkali and adjusted to pH 7.4 by means of HCl. Instead of isolating a purified substance, the authors made a mixture of monomers and oligomers (HpD). The structure of the most active compounds in this mixture has been much debated, and it has been suggested that dihematoporphyrin ether or ester is the most efficient photosensitizer in this mixture. A somewhat purified version was later developed and named Photofrin. This is far from an ideal photosensitizer for use in PDT, due to its composition and difficulty in synthesizing reproducible batches. In addition, HpD has far from optimal spectral properties; a long half-life in the body, and skin and eye photosensitivity for 4-6 weeks (or more) after injection. It is approved, however, for use in many indications, and a large number of patients have been treated with this compound.
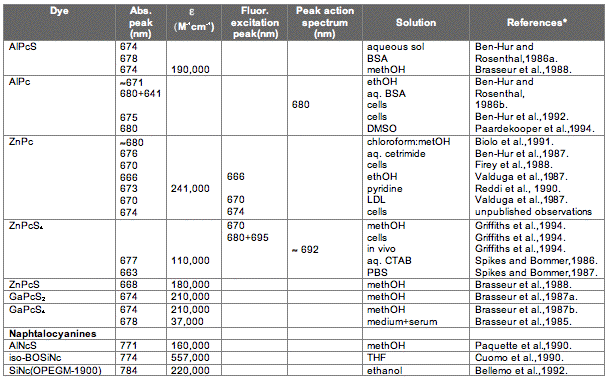
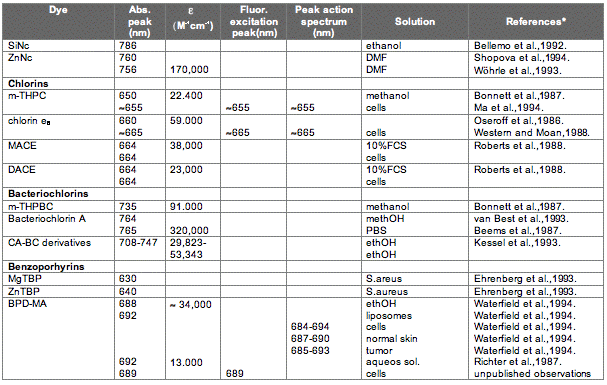
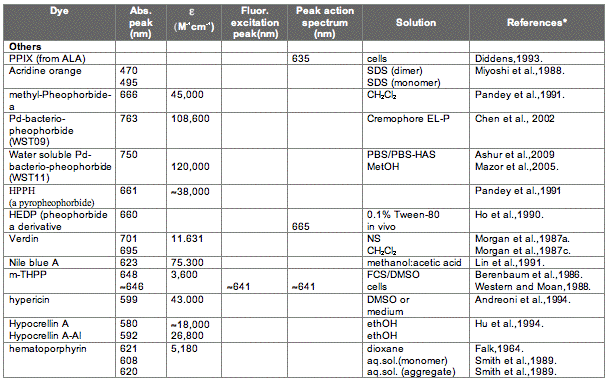
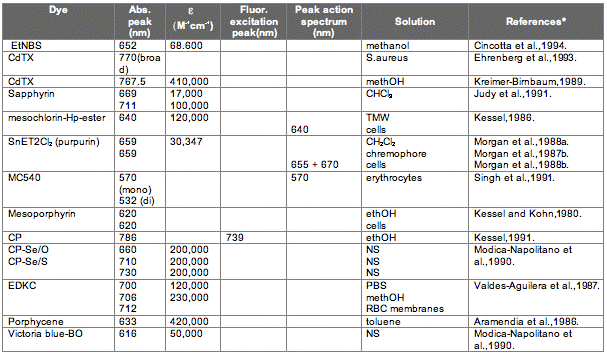
The absorption spectrum of a porphyrin (Figure 5) comprises a Soret band and 4 Q-bands. Naturally occurring porphyrins, like in heme and cytochromes, and melanin are the main chromophores in tissues. If photoactivation of photosensitizers located deep in a tissue is required, activation of the outermost Q-band is necessary, because longer wavelengths of light penetrate tissues better than shorter wavelengths. However, the absorption coefficient of porphyrins is low at this wavelength area. New photosensitizers are being developed with a higher absorption coefficient in this wavelength area and with peak absorption at higher wavelengths [Figures 5-6, Table 1 (at end of module)]. There is an upper limit, however, for how far into the infrared region a photosensitizer can absorb light and still induce singlet oxygen. This upper limit is set by the energy required to excite O2, which is 0.96 eV (22 kcal/mol). The upper wavelength limit is therefore 850-900 nm, depending on the energetic level of the photosensitizers triplet state. The therapeutic window is therefore agreed to be 600-800 nm for in vivo treatment, limited by haemoglobin and melanin in the low wavelength region.
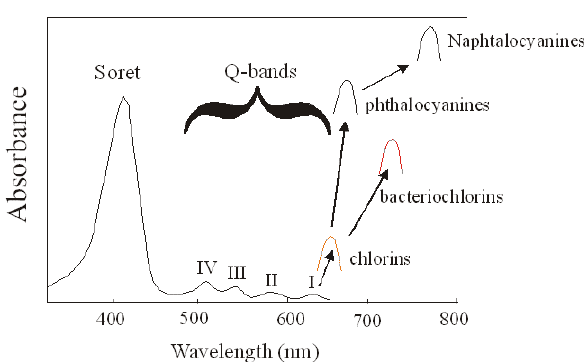
Figure 5. Photosensitizer Absorbance. A full spectrum is shown for a porphyrin-type photosensitizer with its typical Soret-band, and the 4 Q-bands. The main peaks and relative absortion coefficients for photosensitizers developed for PDT are indicated on the figure (not to scale).
The porphyrin-type spectrum is caused by the conjugated ring structure with 22
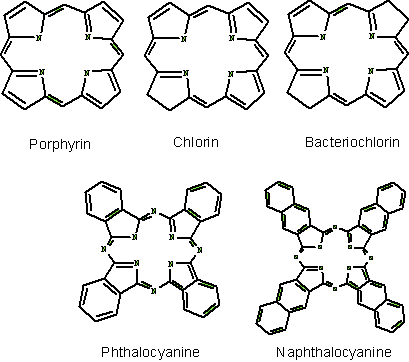
Figure 6. Basic structure of some photosensitizers.
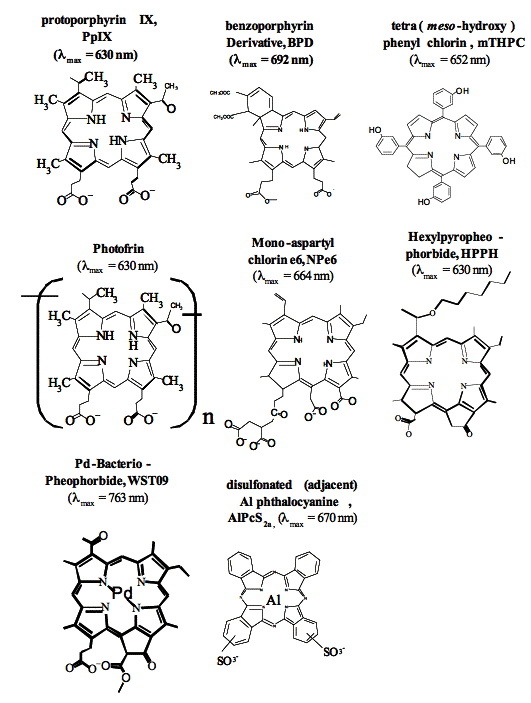
Figure 7. Chemical structures of some clinically used photosensitizes. AIPcS2a is the most efficient component of AIPcSn (Photosense).
There are several other photosensitizers that are variants over these themes, and with side chains that may be beneficial for the compound's pharmacokinetic properties, or other properties as mentioned above. Briefly it should only be mentioned that hydrophobic and amphiphilic phtotosensitzers are generally more efficient in sensitising cells to photoinactivations due to the longer lifetime of singlet oxygen in hydrophobic environments, and easier penetration of such photosensitizers through and into membranes. This is also reflected in all of the photosensitizers that have been approved for clinical use. It should also be mentioned that many photosensitizers may exist as many ionic species in biological environments, and some of these may be hydrophobic.
Endogenously Synthesized and Degraded Photosensitizers
5-Aminolevulinic Acid (5-ALA) is a prodrug for application in PDT and photodynamic diagnosis that has attracted great attention the last 10 years. 5-ALA-based PDT is approved for treatment of actinic keratosis, 5-ALA-methyl ester based PDT is approved for the treatment of actinic keratosis, basal cell carcinoma and Bowen's disease, while 5-ALA-hexyl ester is approved for the destruction of bladder cancer, as described above in the section on The Fluorescence of Photosensitizers. Treatment is based on heme synthesis. A main regulatory step in the heme pathway is linked to 5-ALA synthase activity (Figure 8).
Heme can inhibit the enzyme directly as well as the transcription, translation and transport of the protein into mitochondria. Thus, treatment of cells with 5-ALA overrules the regulatory step of the heme pathway, and induces a high activity through the pathway. Treatment with 5-ALA has been shown to induce the accumulation of porphyrins, mainly protoporphyrin IX (PpIX), which sensitizes cells to photoinactivation. All the intermediates in the heme pathway are porphyrinogens, which are not photochemically active, but protoporphyrinogens are converted to the photosentiser PpIX by means of protophorphyrinogen oxidase, or spontaneously when accumulated in 5-ALA treated cells. Ferrochelatase incorporates Fe2+ into PpIX, and converts it into the photochemically inactive porphyrin heme. 5-ALA may also act as an insecticide and a herbicide in a similar way as used for cancer treatment.
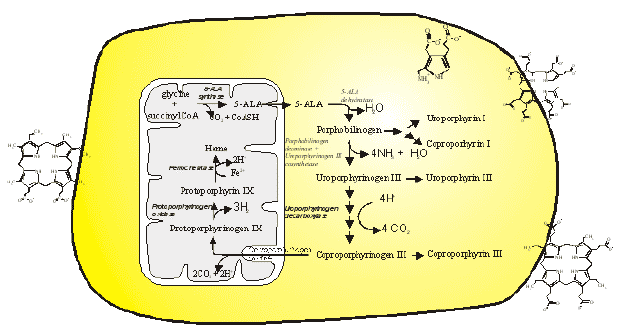
Figure 8. The heme biosynthetic pathway. The synthesis occurs partly in the mitochondria, as indicated on the left side of the figure, and partly in the cytosol. Porphobilinogen, uroporphyrinogen and coproporphyrinogen may undergo enzymatic or autooxidative conversion to photochemically active porphyrins, as indicated on the right side of the figure.
Metabolic errors may cause accumulation of photosensitizers. The most well known example is the group of diseases known as the porphyrias, which cause the accumulation of porphyrin intermediates. The synthesis of porphyrin is described in Figure 8, and porphyrias are caused by lowered activities of one or more of the enzymes in the heme pathway. Porphyrinogens are accumulated and are autooxidized to porphyrins. Several porphyrin-based diseases are described in the literature due to accumulation of different intermediates leading to somewhat different symptoms. Some of the most well known porphyrias are described in Table 2. Although all the cells in porphyria patients are affected, depending on the enzyme defect, mainly erythropoietic cells and liver accumulate large amounts of porphyrins. These inherited diseases are therefore classified as erythropoietic or hepatic, depending on the principal site of expression of the defect.
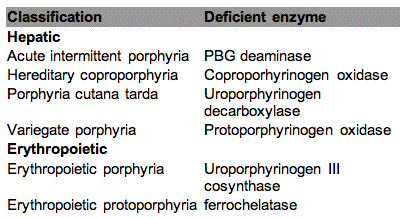
Table 2 . Classification of the major porphyrias.
Several toxins found in plants, fungi and cyanobacteria cause liver damage or dysfunction leading to retention of a degradation product of chlorophyll, phylloerythrin (Figure 9). Chlorophyll is degraded to phylloerythrin by microorganisms in the rumen of sheep, goat and cattle, and phylloerythrin is normally modified by the liver to stimulate excretion through the bile duct. However, certain toxins cause the accumulation of phylloerythrin, which circulates in the blood, accumulates in the skin, and causes damage to the skin and eyes of these animals upon exposure to sunlight. This is a particular problem for sheep in many countries, e.g., geeldikkop caused by the herb Tribulus terrestris has affected several hundred thousand sheep and goats in South Africa (Figure 10).
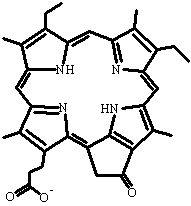
Figure 9. Phylloerythrin.

Figure 10. Examples of damage to animals caused by phylloerythrin accumulation in the skin and light. The pictures were kindly provided by Professor Arne Flåøyen, The Norwegian School of Veterinary Science, Norway.
Non-Porphyrin-Based Naturally Occurring Photosensitizers and Derivatives Thereof
Psoralens have been used in Egypt and India since 1200-2000 BC for the treatment of the common disfiguring disease, vitiligo. Extracts of leaves, seeds, or the root of the plant Ammi majus L. in Egypt and Psoralea corylifolia L. in India were applied directly on the skin, or ingested, and the patients exposed to intense sunlight on these areas. It was later found that the active compounds were psoralens. Purified psoralen was first used in 1974 in combination with UVA light (PUVA), and PUVA is now used for a heterogeneous group of diseases such as vitiligo, psoriasis, and mycosis fungoides (cutaneous T-cell lymphoma). Animals such as cattle and sheep feeding on psoralen-containing plants may suffer from photosensitization. Some insects however do have enzymes to detoxify 8-methoxypsoralens.
The basic structure of psoralen is shown in Figure 11. Many psoralens form adducts with DNA, in particular with thymidine, upon exposure to UV radiation. A photodynamic reaction pathway leading to the formation of singlet oxygen has also been documented, and may be involved in damage to cell membranes as well as DNA. New derivatives of psoralens are under development to improve selectivity for target tissues, and to increase phototoxicity in the target area. They are also under development for viral inactivation of blood products. Gilvocarcins are antitumor antibiotics with a psoralen-resembling aromatic structure that promotes protein-DNA cross-linking when photoactivated by near-UV light.
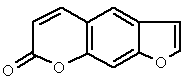
Figure 11. Structure of psoralen (furocoumarin).
Quinones (aromatic organic compounds where an even number of -CH= groups are replaced with -(C=O)- are used in photography, and as dyes, and are widely distributed in plants. Some quinones, like anthraquinones, perylenequinones and hypericin are photosensitizers, and will be described here.
Anthraquinones (AQs, Figure 12) have been used as dyes, antibiotics, solar energy-storing materials and photosensitizers. Some of these compounds have a high yield of triplet states upon excitation by light, and form reactive oxygen species (ROS), including singlet oxygen. The photophysical and photochemical properties of AQs are significantly influenced by their substituents, in particular amino and hydroxyl groups that are in most cases attenuating the
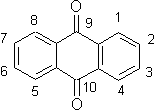
Figure 12. 9,10-Anthraquinone
Anthracyclins, which contain 1.4-dihydroxy-9,10 AQ, represents one of the most prescribed classes of anticancer agents of which doxorubicin (Figure 13) and daunorubicin are the best known. The phototoxicity of these compounds is regarded as a side effect, usually seen in patients subjected to sun exposure. The quantum yield of triplet state formation of these compounds is about 0.2 with a very low
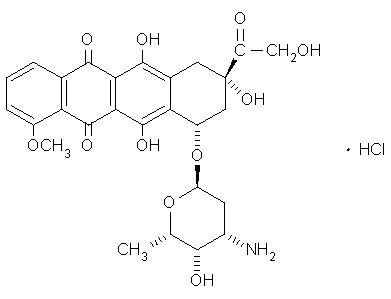
Figure 13. Structure of the anthracycline doxorubicin.
Anthracenediones. Some anthracenediones, i.e., diamino-substituted AQs, have been shown to be good photosensitizers (e.g., 1,5,- and 1,8-diaminoAQs), while others such as the 1,4-diaminosubstituted AQs mitoxantrone and ametantrone, used as anticancer agents, have negligible light activation.
Perylenequinones (PQs) 4,9-dihydroxy-3,10-perylenequinones, of which hypopcrellins (from the parasitic fungi Hypocrella bambuase and Shiraia bambusicola) are the most studied for their photodynamic action (Figure 14). Most of the natural PQs are produced by a large variety of fungi and act as photodynamic phytotoxins of their hosts. PQs are efficient 1O2 generators with quantum yields comparable to those of porphyrins, while O2-. and other ROS are produced to a lesser extent. The main absorption peak of hypocrellins is around 450 nm, but they absorb light of wavelengths up to about 600 nm. Antibacterial (Gram positive) activity has been observed upon activation by light. PQs in combination with light have been shown to be good inhibitors of protein kinase C, a key enzyme in cell proliferation and differentiation.
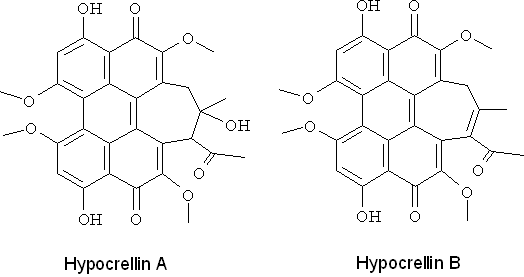
Figure 14. Hypocrellin A and B.
Hypericin (HC), structurally related to PQs, is a well known photodynamic agents (Figure 15). HC is isolated from St.John's wort (Hypericum perforatum). HC is probably the most powerful photosensitizer found in nature, and quantum yields of singlet oxygen formation of about 0.8 have been described. A skin disorder (hypericism) is caused in cattle that ingest large amounts of the Hypericum plant. It acts through both Type I and Type II reactions. Its absorption properties, i.e., in the red wavelength region (peak around 595 nm), make it clinically favorable for treatment of thin cancer lesions, and it has been evaluated clinically for several cancer indications, and for the detection of bladder cancer. HC has antibiotic properties (on gram-positive bacteria), antiviral effects (specific to enveloped viruses, such as herpes simples, cytomegalovirus and HIV, although no clinical benefits have been documented so far), acts as an inhibitor of protein kinase C, and has also been reported as an antidepressant.
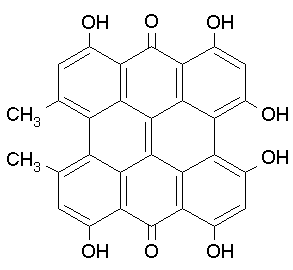

Figure 15. Hypericin and Hypericum perforatum (right side).
Non-Porphyrin-Based Synthetic Photosensitizers
Xanthene (Figure 16) is a heterocyclic ring system, and may be divided into:
• diphenylmethane derivatives (called pyronines)
• triphenylmethane derivatives, consists mainly of phthaleins, and 9-phenylxanthenes (rosamines)
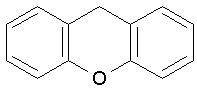
Figure 16. Xanthene.
Phthaleins may be divided into:
• fluoresceins, which contain hydroxygroups
• rhodamines, which contain amino groups
• mixed types containing both amino and hydroxyl groups
Fluoresceins and rhodamines have been shown to be good photosensitizers, and will be described briefly here.
Fluoresceins. Figure 17 shows the general structure of the fluorescein group.
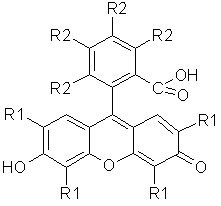
Figure 17. Structure of fluoresceins. The R1 and R2 groups consist of only hydrogen in fluorescein, while in rose Bengal R1 is I, and R2 Cl, and in eosin R1 is Br, and R2 H.
Eosin was the first photodynamic type photosensitizer tested by Oscar Raab in 1900. Fluorescein is a poor photosensitizer, but by replacing some of the hydrogens with halides, these compounds become efficient photosensitizers (Figure 17). Rose Bengal is often used as a generator of 1O2 (
Rhodamines. The most used and studied rhodamine is rhodamine 123 (Rh123, Figure 18). It is much used as a mitochondrial stain. This localization is due to its delocalised positive charge, which traps Rh123 in the negatively charged matrix of the mitochondria. It has a high quantum yield of fluorescence (
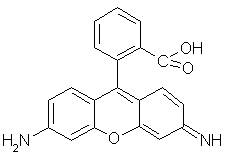
Figure 18. Structure of rhodamine 123.
The quantum yield for 1O2 formation is dramatically improved by bromination of Rh123 (e.g., tetrabromo Rh123), enhancing the
Cyanine dyes contain two heterocyclic systems connected by a bond between nuclear carbon atoms (apocyanines). The two hetereocycles can be linked through methine groups, i.e., monomethines (cyanines) or polymethines (polycarbocyanines). These compounds were originally used in photographic emulsions, but have later been found to be good photosensitizers, and have been explored for diagnostic and therapeutic purposes. The most used cyanine dyes for phototherapeutic purposes are merocyanine 540 (MC540), kryptocyanine and chalcogenapyrylium. The absorption properties of these compounds (
MCD540 (Figure 19) has a high selectivity and affinity for leukemia, lymphomas and neuroblastoma in autologous bone marrow grafts, and has undergone clinical trials for these purposes. The phototoxicity of MC540 is due to a photodynamic process involving 1O2. MC540 PDT targets membrane lipids, especially in the plasma membrane. Pre-exposure of MC540 to light leads to photoproducts that inhibit topoisomerase II, a main target in cancer therapy.
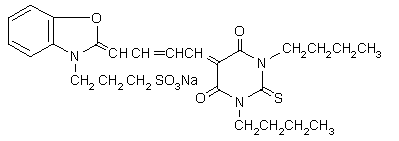
Figure 19. MC540
Kryptocyanines (carbocyanines, Figure 20) have a delocalised positive charge, and will, as does Rh123, penetrate through membranes due to their hydrophobic character, and concentrate in the negatively charged mitochondrial matrix. The yield of triplet state and 1O2 formation of these dyes is low. Internal conversion from the first excited state to the ground state is efficient, and the heat produced may generate a phototoxic effect. N,N'-bis(2-ethyl-1,3-dioxolane)kryptocyanine (EDKC) has shown interesting phototherapeutic properties, but little research has been performed recently.
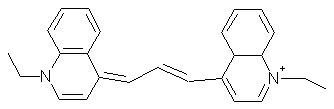
Figure 20. Kryptocyanine.
Chalcogenapyrylium dyes (Figure 21) are also cationic, and target the mitochondria preferentially. Se/Te chalcogenapyrylium has been most studied, and has an absorption maximum at 770 nm, with a relatively low
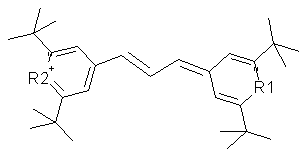
Figure 21. Chalcogenapyrylium dyes, where O, Se or Te have been used in positions R1 and R2.
Triarylmethane Dyes. Triarylmethane photosensitizers are cationic dyes related to the cyanines and exhibit properties similar to these. Victoria blue-BO (VBBO, Figure 22) is the best studied and has been shown to target mitochondrias.
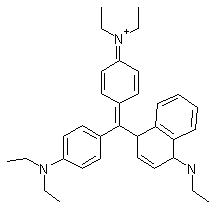
Figure 22. Victoria Blue BO
Phenothiazines, Phenoxazines and Acridines. The structures of phenothiazine and phenoxazine are shown in Figure 23. The phenothiazines dye methylene blue (MB) is widely used as a vital stain (as is also the case for toluidine blue (TBO)), in diagnosis and as a tumor marker during surgery. MB and TBO are also under development for pathogen reduction in blood products (MB is currently used by the Swiss and German Red Cross for this purpose), and bacterial eradication in dental plaques. The eradication of gram-negative bacteria is very efficient with TBO, probably due in part to its positive charge, which seems to be a prerequisite for the efficient photodynamic eradication of such bacterias. A large variety of phenothiazines have been synthesized, of which MB, TBO, azure A-C and thionin are the most well known. Both phenothiazines and phenoxazines are good producers of 1O2, but other reactive species seems also to be involved in the photodynamic activity of these compounds.
Of the phenothiazines, only methylene blue has been evaluated clinically on inoperable oesophageal tumors by intratumoral injection of the photosensitizer. Derivatives of methylene blue are undergoing clinical trials for the treatment of chronic skin ulcers. Phenothiazines, phenoxazines and acridine cationic dyes accumulate in acidic vesicles like the lysosomes, while the cyanine cationic dyes accumulate mainly in the mitochondria. This pattern may, however, be influenced by the side chains on the compounds and the extracellular concentration. These compounds have also high affinity for DNA by intercalating into the double strand.
Some of these compounds (acridine orange (AO), TBO, pyronine Y) also bind RNA and damage RNA in a photodynamic manner, involving the formation of 1O2. It should be noted that phenothiazines and phenoxazines (at least MB and TBO) are reduced intracellularly by reducing agents such as NADH and FADH2, forming leuco MB/TBO, which is colourless and photodynamiclly inactive. The phenothiazines and phenoxazines have favourable spectral properties for therapeutic utilization with strong absorption in the 600-660 nm range. Nile blue has very low
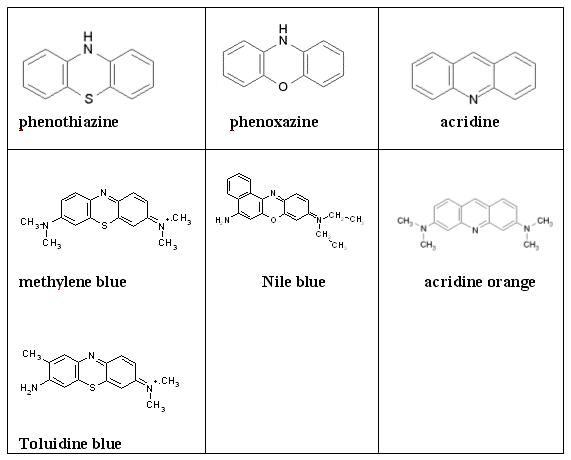
Figure 23. Chemical structure of phenothiazines, phenoxazines and acridines and some examples of regularly used dyes of these classes of compounds.
Chemicals that Cause Unwanted Biological Effects upon Exposure to Light
Several widely used medicaments have photosensitizers properties, which may cause skin reactions upon exposure to light. Most of these compounds are di- or tricyclic aromatic molecules, which are activated by UVA or UVB light. The photosensitization reactions may be due to the drugs themselves, their metabolites, and toxic reactions may be caused by the photoproducts. These drugs may cause dermatitis, erythema, bleb formation, itching and burning sensation during sun exposure. Examples of such photosensitiers are quinolones, tetracyclins, sulfonamides, phentiazine derivatives (antihistamines), non-steroid ant-inflammatory agents (NSAIDs), and chloroquine. Uroporhyrin has been shown to accumulate after treatment with steroids, antibiotics, hypnotics and sedatives. Some anthraquinone dyes used in textile industry may cause skin reactions known as bikini dermatitis. Cosmetic products (cologne, perfumes) that contain naturally occurring furanocoumarins, may induce a skin condition known as berloque dermatitis. Exposure to sunlight may cause phototoxic reactions leading to pigmentation, which may last for a long time. It should also be cautioned that medical drugs can undergo photochemical reactions before use, and cause attenuated clinical responses or unexpected side effects.
SUMMARY
A large number of photosensitizers have been evaluated for potential clinical use. Many of these photosensitizers absorb light below 600 nm, thus limiting their therapeutic use to superficial lesions. The treatment of thicker tissue layers requires photosensitizers that absorb light above 600 nm. A large number of photosensitizers with such a property have been developed in the last 20 years, and are currently being evaluated in clinical trials, mainly for various cancers. The specificities of these photosensitizers to target lesions are still too low, and current and future research is focused on technologies to solve this problem.
Suggested Readings
Ali H, van Lier JE. (1999) Metal complexes as photo- and radiosensitizers. Chem. Rev. 99:2379-450.
Bonnett,R. (1999) Photodynamic therapy in historic perspective. Rev.Contemp.Pharmacother. 10: 1-17.
Brown SB, Brown EA, Walker I.(2004) The present and future role of photodynamic therapy in cancer treatment. Lancet. Oncol. 2004 5:497-508.
Corash, L. (2001) Inactivation of infectious pathogens in labile blood components: meeting the challenge. Transfus. Clin. Biol.8:138-45.
Kagan,J. (1993) Organic photochemistry. Principles and applications. Academic Press.
MacDonald,I.J. and Dougherty,T.J. (1999) Basic principles of photodynamic therapy. J. Porphyrins and Phthalocyanines. 5:105-129.
Morgan J, Oseroff AR. (2001) Mitochondria-based photodynamic anti-cancer therapy. Adv. Drug Deliv. Rev. 49:71-86.
O'Connor AE, Gallagher WM, Byrne AT. (2009) Porphyrin and nonporphyrin photosensitizers in oncology: preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 85:1053-74.
Redmond, R.W. and Gamlin, J.N. (1999) A compilation of singlet oxygen yields from biologically relevant molecules. Photochem. Photobiol. 70:391-475.
Roberts JE. (2002) Screening for ocular phototoxicity. Int. J. Toxicol. 21:491-500.
Theodossiou TA, Hothersall JS, De Witte PA, Pantos A, Agostinis P (2009) The Multifaceted Photocytotoxic Profile of Hypericin. Mol. Pharm. e-pub
Tønnesen,H.H. (2001) Formulation and stability testing of photolabile drugs. Int. J. Pharmaceutics. 225: 1-14.
Sobolev AS, Jans DA, Rosenkranz AA. (2000) Targeted intracellular delivery of photosensitizers. Prog. Biophys. Mol. Biol. 73:51-90.
Wainwright M, Mohr H, Walker WH. (2007) Phenothiazinium derivatives for pathogen inactivation in blood products. J. Photochem. Photobiol. B. 86:45-58.
Wainwright,M. (2000) Methylene blue derivatives - suitable photoantimicrobials for blood product disinfection? Int. J. Antimicrob. Agents. 16: 381-394.
Zarebska Z, Waszkowska E, Caffieri S, Dall'Acqua F. (2000) PUVA (psoralen + UVA) photochemotherapy: processes triggered in the cells. Farmaco. 55:515-20.
[ Return to Text ]
11/11/09