MOLECULAR TARGETS OF PHOTOSENSITIZATION
Some Biological Chemistry of Singlet Oxygen (1O2)
Garry R. Buettner
Free Radical and Radiation Biology & ESR Facility
Med Labs B180, The University of Iowa
Iowa City, IA 52242
garry-buettner@uiowa.edu
When a component of a system (a photosensitizer) absorbs radiant energy, especially light, a resultant action is called photosensitization. This action can range from chemical changes in target molecules to biological responses in organisms.
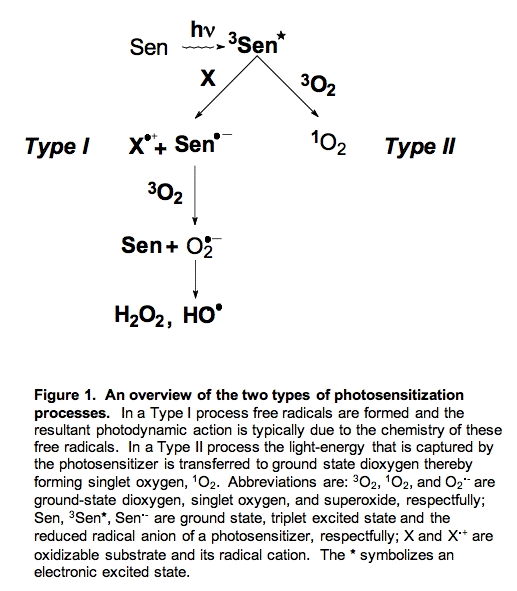
There are two major pathways for photosensitization, Type I and Type II (Figure 1). In Type I photosensitization the excited photosensitizer (3Sen*) abstracts an electron/hydrogen atom from another component of the system and two radicals are formed, a photosensitizer radical (Sen.-) and a substrate radical (X.+). If oxygen is present, the photosensitizer radical can transfer the newly acquired electron to oxygen forming superoxide radical (O2.-); the photosensitizer (Sen) returns to its ground state. Thus, in Type I reactions chemical changes occur and new products are formed (e.g., X.+ and O2.-).
In Type II reactions the photosensitizer transfers its excitation energy to ground state dioxygen resulting in singlet oxygen (1O2) and ground state photosensitizer. Singlet oxygen can release this energy in the form of heat, a process called physical quenching or it can react with electron-rich compounds such as unsaturated fatty acids resulting in hydroperoxides. A reaction of 1O2 that results in the net depletion of dioxygen (O2) is called chemical quenching.
II. What is singlet oxygen?
A. Dioxygen, O2 or 3O2. The oxygen you are breathing is officially called dioxygen (or dioxidanidyl). In contrast to the vast majority of molecules, dioxygen in its ground state has two unpaired electrons (Figure 2). Because there are two unpaired electrons, dioxygen is a triplet state molecule. A triplet state implies there are three ways to quantum mechanically describe the molecule.
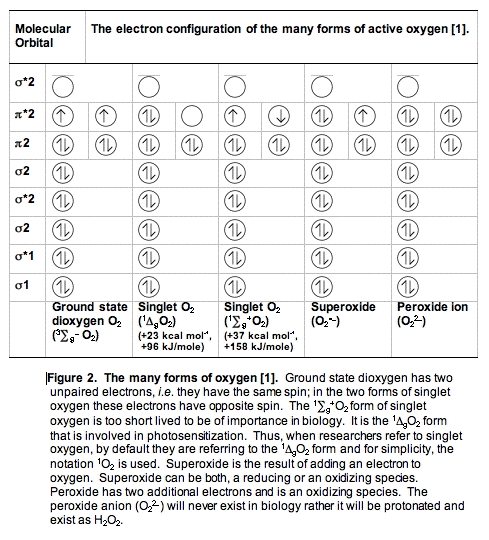
To react with another molecule the electrons in ground-state dioxygen need to be spin paired, a process that requires energy. The activation energy for most reactions of oxygen is relatively high (23 kcal/mole, 96 kJ/mole), making the non-catalyzed reactions of dioxygen very slow at physiological temperatures. (A fire needs a spark to get started.) It is because of this high activation energy for reactions of dioxygen that life exists (otherwise we would burn up spontaneously).
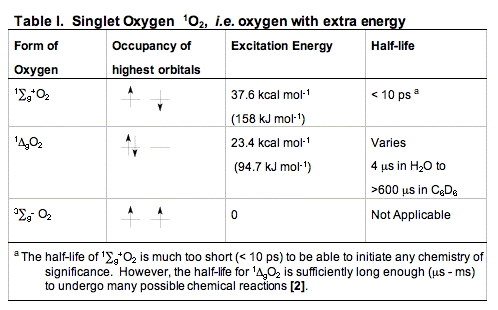
B. Singlet Oxygen, 1O2. When dioxygen has excess energy, the two unpaired electrons in the outer orbital can pair up. When this happens we have singlet oxygen. There are two forms of singlet oxygen (Figure 2). However, there is but a single way to quantum mechanically describe each of these forms of 1O2. The 1
 g + O2 form of singlet oxygen is too short-lived to be of importance in biology (Table I). It is the 1
g + O2 form of singlet oxygen is too short-lived to be of importance in biology (Table I). It is the 1 gO2 form that is involved in photosensitization. Singlet oxygen is not a free radical because all electrons are spin-paired. It is electrophilic; it has essentially the energy of activation needed for reactions; thus, 1
gO2 form that is involved in photosensitization. Singlet oxygen is not a free radical because all electrons are spin-paired. It is electrophilic; it has essentially the energy of activation needed for reactions; thus, 1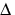 gO2 reacts readily with electron-rich molecules. Targets for oxidation by 1O2 would be lipids, amino acids and nucleic acids that have double bonds as well as sulfur-containing amino acids.
gO2 reacts readily with electron-rich molecules. Targets for oxidation by 1O2 would be lipids, amino acids and nucleic acids that have double bonds as well as sulfur-containing amino acids.
C. Quenching of 1O2. Quenching is a term that has many meanings: to eliminate, to bring to and end, to destroy, or to extinguish the fire. In the photosciences quenching implies the removal of the excitation energy in a species or the actual destruction or removal of a species. Thus, to quench 1O2 is to remove it. There are two types of quenching possible:
i. Physical quenching is the removal of the excitation energy from 1O2 without any chemical changes. The removal of this energy will return the oxygen molecule to its ground state energy, thus dioxygen reappears in the system and no harm is done. The energy is converted to heat, but the amount is so small that in most circumstances no detectable change in temperature will be seen.
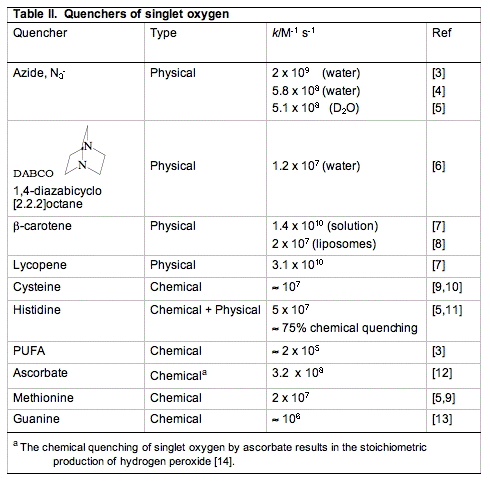
Common tools used to physically quench 1O2 are azide (N3-) and hindered amines such as DABCO (Table II). In biological settings the carotenoids are outstanding physical quenchers of singlet oxygen. Compounds like β-carotene and lycopene are able to remove the excitation energy in 1O2 without being damaged themselves. This quenching is essential for the protection of structures that range from plants to skin and eyes.
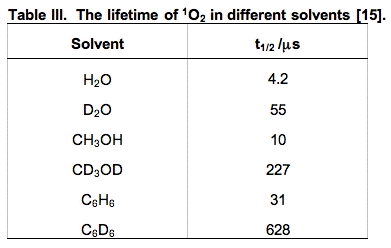
ii. Chemical quenching is a term used to signify that an actual chemical reaction has occurred. These oxidation reactions are what make 1O2 so damaging. Compounds that have areas of high electron density are susceptible to chemical oxidation by 1O2. In biology, these targets are in general unsaturated lipids, nucleic acids and amino acids, especially sulfur-containing amino acids. The short lifetime of 1O2 limits the rate of its reactions. However, its lifetime is solvent-dependent. In general it is short-lived in protic solvents and long-lived in non-protic and deuterated solvents (Table III). In living systems, the reactivity of 1O2 will be different in membranes compared to aqueous regions of cells. In general, 1O2 will have a longer lifetime in membranes, resulting in a greater chance of chemical reactions.
III. Singlet oxygen reacts with double bonds:
Singlet oxygen reacts with double bonds, but the exact reaction depends on the nature of these double bonds. There are three main types of addition reactions as well as potential electron transfer reactions:
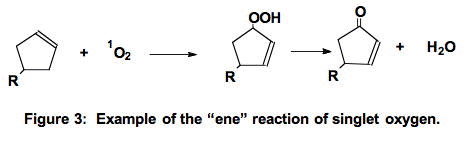
A. The "ene-reaction" [16]. Singlet oxygen can add to a carbon of a double bond. In the "ene" reaction (Figure 3) the double bond moves to neighboring carbons and a hydroperoxide is produced. Note that this is not a free radical process.
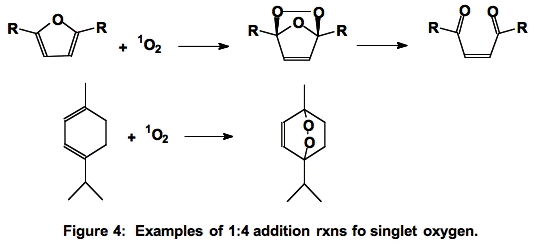
B. Diels-Alder Type Addition. In the 1:4 addition reaction, singlet oxygen adds across two double bonds forming an endoperoxide
(Figure 4).
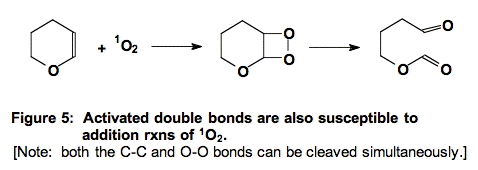
C. Addition to Activated Double Bonds (Figure 5).
D. Electron Transfer: 1O2 + e- → O2.-
Because singlet oxygen is a an effective oxidant it can undergo both a one-electron reduction and a two-electron reduction. The one-electron reduction potential of 1O2 to form superoxide is +650 mV, pH 7 [17]. Thus many biological reductants can in principle facilitate this reaction.
1O2 + 2e- +2H+ → H2O2
Ascorbate (vitamin C) readily serves as both a one-electron and two-electron reducing agent; ascorbate does not readily react with oxidants to form covalent bonds. Thus, in the presence of ascorbate, it might be expected that a fraction of 1O2 might be reduced by one-electron to form superoxide; it is a highly favorable reaction
(ΔE = +650 (1O2/O2•−) - (+280 (Asc.- /AscH-)) = +370 mV [17]. However, the reaction of ascorbate with 1O2 appears to result in the stoichiometric production of H2O2 [14]. Although the detailed mechanisms are not known, the overall reaction appears to be:
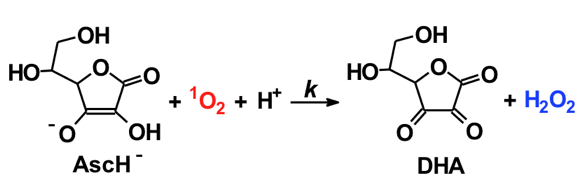
The reaction is rapid, k = 3.1 x 108 M-1 s-1 for the reaction of the ascorbate monoanion with 1O2 [12]. (The first pKa of ascorbic acid is approximately 4.0, the second is 11.3; thus, the chemistry of ascorbate in biology is dominated by the chemistry of the ascorbate monoanion.) As seen in Table II, ascorbate is by far the most efficient chemical quencher of 1O2. The very fast reaction of ascorbate with 1O2 and its high concentration in the water space of cells suggest that it could be an important sink for 1O2 in vivo. The product of the reaction is H2O2, another oxidant. Hydrogen peroxide will have a much greater diffusion distance than 1O2 generating a different type of oxidative challenge for cells and tissues.
IV. How Does 1O2 React with Lipids, Proteins and DNA?
A. DNA and 1O2. Because of its redox potential guanosine (2'-dG) is the DNA base that is most easily oxidized and consequently the most reactive with singlet oxygen. As seen below, the reaction of 1O2 results in the oxygenation at carbon-8. This product, 8-hydroxy-2’-deoxyguanosine (formally called 7,8-dihydro-8-oxo-2’-deoxyguanosine or for short 8-OHdG), is the same product formed upon oxidation by hydroxyl free radical (Figure 6).
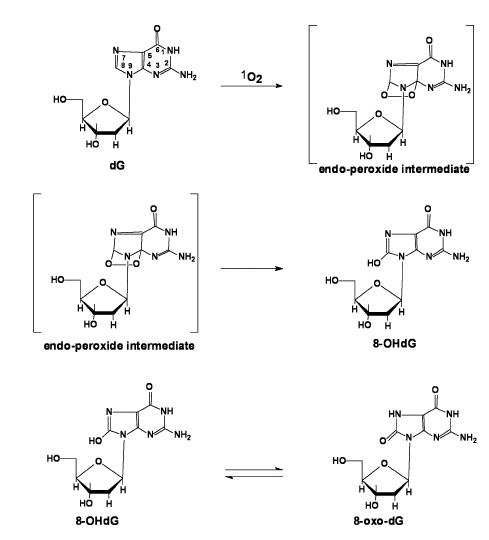
Figure 6: Pathway to formation of 8-OHdG / 8-oxo-dG.
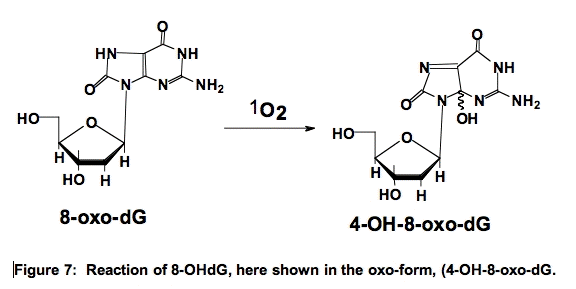
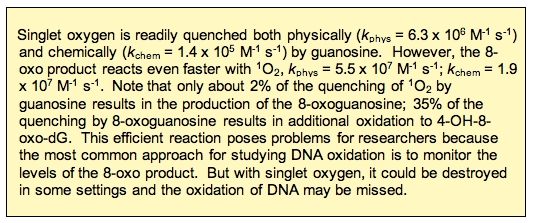
Interestingly, 8-OHdG is 100-times more reactive with 1O2 than 2’-dG [18]. The reaction of 8-OHdG results in the formation of 4,8-dihydro-4-hydroxy-8-oxo-2’-deoxyguanosine (4-OH-8-oxo-dG) (Figure 7) [19,20]. These oxidations in DNA can pose problems in an organism.
B. Proteins and 1O2. The susceptible proteins are those with areas of high electron density because of double bonds or sulfur moieties. These are cysteine, methionine, tryptophan, tyrosine and histidine.
a. Cysteine. Thiols have relatively high electron density and thus react readily with 1O2. The kinetically favored reaction is with the deprotonated thiol, for cysteine thiolate, k = 1.5 x 108 M-1 s-1; for the protonated thiol, k < 4 x 104 M-1 s-1; the overall observed rate constant at pH 7 is kobs = 8.9 x 106 M-1 s-1 [10].
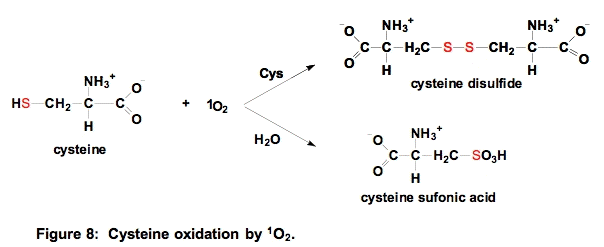
If the concentration of sulfhydryl compounds is relatively high, then a disulfide will be formed. If the concentration of thiols is low, then sulfonic acids are produced [21]. The reaction shown above can be generalized to all sulfhydryls and the disulfide that results could be a mixed disulfide [22].
b. Methionine (Met). Methionine residues are often essential for the activity of proteins. Oxidation of methionine residues by 1O2 can produce methionine sulfoxide (Met-SO) that can further oxidize to methionine sulfone
(Figure 9).
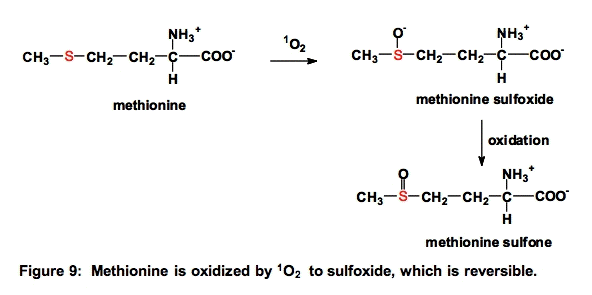
The oxidation of methionine to methionine sulfoxide and cysteine to disulfides are the only ROS-mediated modifications of proteins that can be directly reversed, i.e., by enzymes [22].
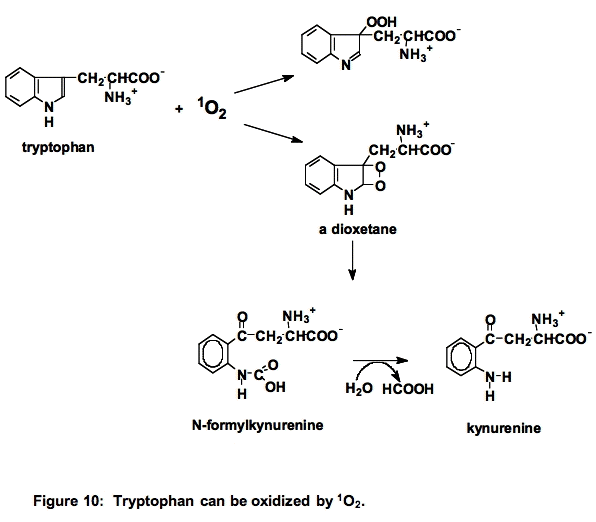
c. Tryptophan (Trp). Tryptophan is a powerful singlet oxygen scavenger, k ≈ 108 M-1 s-1. Reaction with singlet oxygen can result in cleavage of the indole ring (Figure 10) [23]:
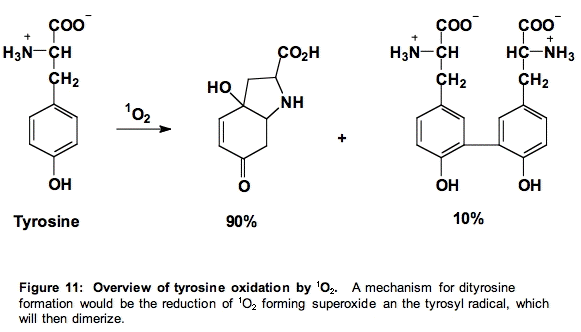
d. Tyrosine (Tyr). The products of the reaction of tyrosine with singlet oxygen are those expected from the reactions of the tyrosyl radical. Thus, it appears as if singlet oxygen oxidizes tyrosine to form superoxide and the phenoxyl radical of tyrosine (Figure 11) [24].
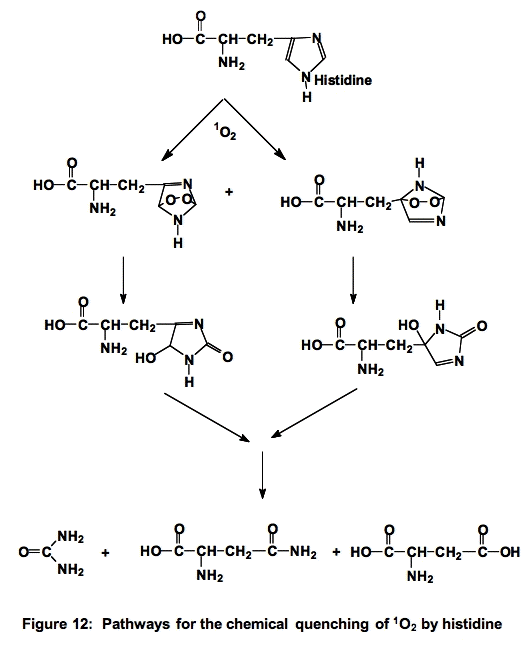
e. Histidine (His). The reactions of histidine with 1O2 have been the subject of much study. Histidine quenches singlet oxygen both physically and chemically (≈5 x 107 M-1 s-1 [3,5,9]) , ≈ 75% is chemical quenching, as illustrated below (Figure 12). It is thought that histidine is one of the major targets for singlet oxygen attack.
C. Lipids and 1O2. Lipids can also be a target of 1O2-mediated oxidation. Because of the electrophilic nature of singlet oxygen, it will only react with unsaturated lipids, including cholesterol that are subject to this mode of oxidation.
i. PUFA Oxidation by 1O2
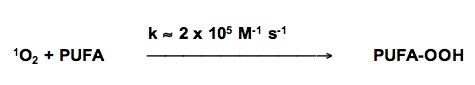
Singlet oxygen reacts with unsaturated lipids to form lipid hydroperoxides, but the mechanism is different than free radical-mediated oxidations. However, the hydroperoxides formed are highly reactive and with the aide of ferrous iron can play an important role in initiating free radical chain reactions (Figure 13).
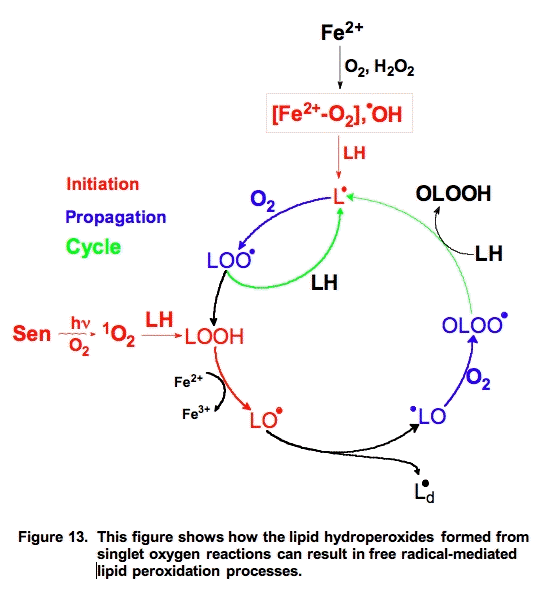
The actual lipid hydroperoxides formed from free radical-mediated lipid peroxidation and singlet oxygen addition reactions are different (Figure 14). Both processes result in the movement of a double bond. In the free radical process, the double bond shifts in such a way to result in a conjugated system. These conjugated double bonds have a significant absorption (ε230-240 = 28,000 M-1 cm-1 [25]) that can be used to monitor the overall progress of a peroxidative process [26].
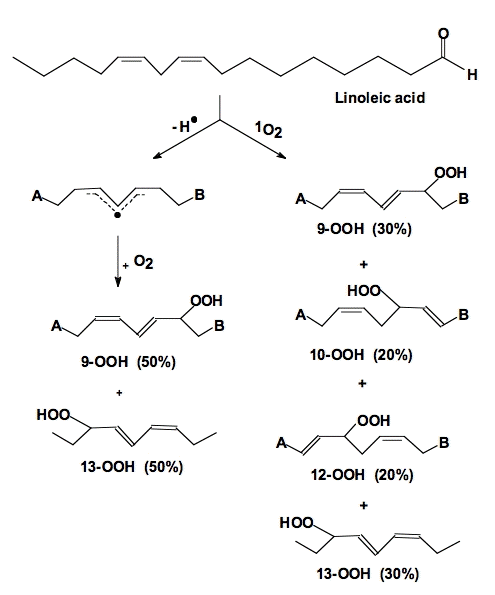
Figure 14. Oxidation of linoleic acid. When linoleic acid is oxidized by free radical processes, dioxygen adds to either carbon-9 or carbon-13. In homogeneous solution a 50:50 mixture of 9-OOH and 13-OOH results; the double bonds shift to produce a conjugated diene. However, when 1O2 reacts with these same double bonds, it can also add to carbons 10 and 12. The double bonds shift in a way that does not produce conjugated dienes [27].
Singlet oxygen reacts with the double bonds of unsaturated fatty acids in an "ene" addition reaction, Figure 14. However, when the double bond shifts the result need not be a conjugated system. Thus, it is usually not appropriate to examine 1O2-mediated lipid peroxidation by monitoring conjugated diene formation.
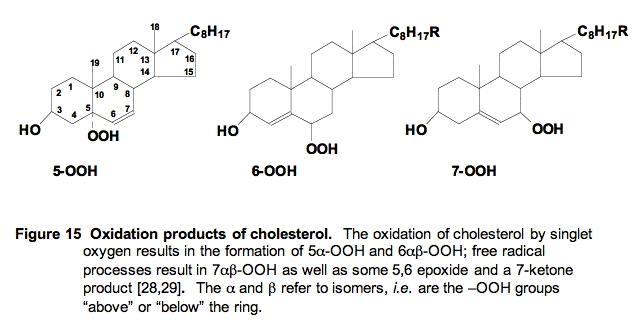
ii. Cholesterol Oxidation by 1O2. Cholesterol can also be oxidized by both free radical and singlet oxygen processes. However, the products are quite different (Figure 15); monitoring them has added to our understanding of the mechanisms of cellular lipid peroxidation. Free radical attack results in formation of a hydroperoxide and later alcohol at the 7-position, while singlet oxygen results in the formation of hydroperoxides and alcohols at the 5- and 6-positions.
V. Cellular and Tissue Targets of 1O2.
To predict the important targets that may be oxidized when 1O2 is produced in cells and tissues three things must be considered:
1. the rate constant of the reaction of 1O2 with the target;
2. the concentration of the target; and
3. the ability of the cell to deal with oxidative damage on this target.
Reaction rate constants for the reactive moieties in some of the principal quenchers of 1O2 in a cell are presented in Table IV. These rate constants have a wide range. The importance of each reaction cannot be judged by the magnitude of the rate constant alone. To get a better idea of the probability of 1O2 being quenched by each possible target, the concentration of the competing species in a cell must be considered.
If the cellular concentration of these quenchers are multiplied by the second-order rate constant for their reaction with 1O2, the pseudo first-order rate constants that result (k') provide a good indicator of the overall quenching of 1O2 done by each species. The rate equations will have the form:
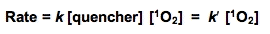
where k' = k [quencher] is a pseudo first-order rate constant. If we assume that [1O2] is the same for each quencher, then a comparison of k' for the various quenchers will allow a direct comparison of their relative rates of quenching singlet oxygen.
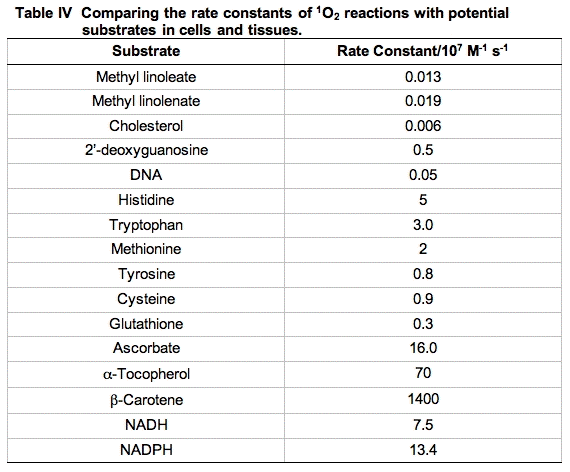
An excellent starting point to make these comparisons has been provided by a study using murine L-1210 leukemia cells [30]. The cells were dispersed by detergent and the total quenching of 1O2 was determined. Then using measurements of the concentration of the principal components of the cell and the rate constants for quenching of 1O2 by these materials, pseudo first-order rate constants were determined and compared (Table V).
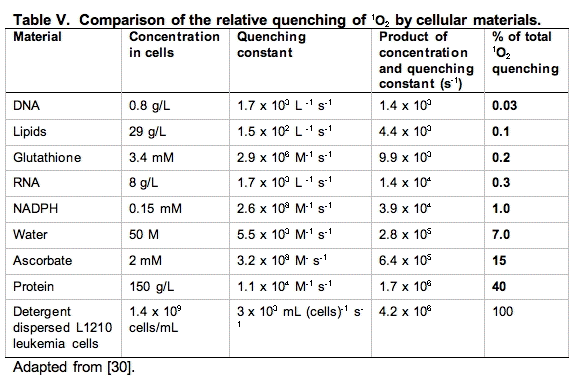
It is clear from Table V that the principal target of singlet oxygen in cells are the cellular proteins. From a kinetic point of view, it would appear DNA is not an important target. However, it must be kept in mind that even though only a small fraction of the 1O2 reacts with DNA, if this is not repaired and it is in a critical region, it could be devastating. Likewise, damage to key proteins can leave the cell severely compromised. Thus, these types of studies are very informative, but the information provided must be blended with other information to gain perspective on the actions and targets of singlet oxygen.
VI. Singlet Oxygen and Health Issues.
Singlet oxygen is now known to be the key chemical species produced in skin when exposed to visible light leading to skin-damage for people suffering from porphyrias. Help for these people lies in increasing the content of β-carotene in their skin. As seen in Table II, β-carotene is one of the most efficient physical quenchers of 1O2, thereby providing protection for the skin from singlet oxygen oxidations.
β-Carotene is also a key to protecting plants from singlet oxygen formed by components of the photosynthetic system. This protection is probably one of the key things missing when plants suddenly encounter high light environments after a long time in low-light settings.
Singlet oxygen is the key oxidizing species produced by photosensitizers used in the photodynamic therapy of cancer. This cancer therapy is in its infancy and as the technical aspects are improved it will become widely used in the treatment of cancer.
Singlet oxygen appears to be a major species produced in cells by UVA (320 - 400 nm), a key component of sunlight. UVA appears to result in oxidations in the cell, while UVB (290 - 320 nm) is detrimental because of the damage done to DNA.
Thus, singlet oxygen is a major concern for health issues related to exposure to light; it is used for good in photodynamic therapy (PDT), but is detrimental because of its ability to oxidize a wide variety of biological targets.
VII. References
1. Laing M. (1989) The three forms of molecular oxygen. J Chem Ed. 66:453-455.
2. Weldon D, Poulsen TD, Mikkelsen KV, Ogilby PR. (1999) Singlet sigma: the "other" singlet oxygen in solution. Photochem Photobiol. 70: 369-379.
3. Wilkinson F, Helman WP, Ross AB (1995) Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation. J Phys Chem Ref Data. 24:663-1021.
4. Hall RD. Chignell CF. (1987) Steady-state near-infrared detection of singlet molecular oxygen: a Stern-Volmer quenching experiment with sodium azide. Photochem Photobiol. 45:459-464.
5. Lindig BA, Rodgers MAJ. (1981) Rate parametes for the quenching of singlet molecular oxygen by water-soluable and lipid-soluable substrates in aqueous and micellar systems. Photochem Photobiol. 33:627-634.
6. Ogryzlo EA, Tang CW. (1970) Quenching of oxygen by amines. J Am Chem Soc. 92:5034-5036.
7. DiMascio P, Kaiser S, Sies H. (1989) Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch Biochem Biophys. 274:532-538.
8. Fukazawa K (2000) Singlet oxygen scavenging in phospholipid membrane. Meth Enzymol. 319:101-110. Meth Enzymol. 319:101-110.
9. Michaeli A, Feitelson J. (1994) Reactivity of singlet oxygen toward amino acids and peptides. Photochem Photobiol. 59:284-298.
10. Rougee M, Bensasson RV, Land EJ, Pariente R. (1988) Deactivation of singlet molecular oxygen by thiols and related compounds, possible protectors against skin photosensitivity. Photochem Photobiol. 47:485-489.
11. Michaeli A, Feitelson J. (1994) Reactivity of singlet oxygen toward amino acids and peptides. Photochem Photobiol. 59:284-299.
12. Bisby RH, Morgan CG, Hamblett I, Gormahn AA. (1999) Quenching of singlet oxygen by trolox C, ascorbate and amino acids: Effects of pH and temperature. J. Chem. Phys. A. 103:7454 - 7459.
13. Notre Dame Radiation Laboratory. NDRL Radiation Chemistry Data Center. [web site]
14. Kramarenko GG, Hummel SG, Martin SM, Buettner GR. (2006) Ascorbate reacts with singlet oxygen to produce hydrogen peroxide. Photochem Photobiol. 82: 1634-1637.
15. Rodgers MAJ. (1984) Activated oxygen. In, Primary Photo-Processes in Biology and Medicine. Ed Bensasson RV, Jori G, Land EJ, Truscott TG. NATO ASI Series A, Life Sciences Vol 85, pp 181-195.
16. Harding LB, Goddard WA. (1980) The mechanism of the ene reaction of singlet oxygen with olefins. J Am Chem Soc. 102:439-449.
17. Buettner GR. (1993) The pecking order of free radicals and antioxidants: Lipid peroxidation,
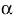 -tocopherol, and ascorbate. Arch Biochem Biophys. 300:535 543.
-tocopherol, and ascorbate. Arch Biochem Biophys. 300:535 543.
18. Sheu C, Foote CS. (1995) Reactivity toward singlet oxygen of a 7,8-dihydro-8-oxoguanosine ("8-hydroxyguanosine") formed by photooxidation of a guanosine derivative. J Am Chem Soc. 117:6439-6442.
19. Devasagayam TPA, Steenken S, Obendorf MSW, Schulz WA, Sies H. (1991) Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 30:6283-6289.
20. Cadet J, Ravanat LJ, Buchko HC, Ames BN. (1994) Singlet oxygen DNA damage: chromatographic and mass spectrometric analysis of damage products. Meth Enzymol. 234 Part D:79-88.
21. Buettner GR, Hall RD. (1987) Superoxide, hydrogen peroxide and singlet oxygen in hematoporphyrin derivative-cysteine, -NADH and -light systems. Biochim Biophys Acta 823: 501-507.
22. Stadtman ER, Barlett BS. (1997) Free radical-mediated modification of proteins. In Free Radical Toxicology. Edited by Wallace KB, CRC Press Boca Raton, ISBN:1560326328, pp71-87.
23. Maskos Z, Rush JD, Koppenol WH. (1992) The hydroxylation of tryptophan. Arch Biochem. Biophys. 296: 514-520.
24. Jin F, Lietich J, von Sonntag C. (1995) The photolysis (λ=254 nm) of tyrosine in aqueous solutions in the absence and presence of oxygen. The reaction of tyrosine with singlet oxygen. J Photochem Photobiol A. 92:147-153.
25. Chan HWS, Levitt G. (1977) Autoxidation of methyl linoleate: Separation and analysis of isomeric mixtures of methyl linoleate hydroperoxides and methyl hydroxylinoleates. Lipids. 12:99-104.
26. Bors W, Erben-Russ M, Michel C, Saran M. (1990) Radical mechanisms in fatty acids and lipid peroxidation. Free Radicals, Lipoproteins and Membrane Lipids Ed. A Crastes de Paulet et al. Plenum Press, New York.
27. Frankel EN. (1998) Photooxidation of unsaturated fats. In: Lipid Oxidation. Chapter 3. pp. 43-54. The Oil Press LTD, Dundee, Scotland.
28. Korytowski W, Girotti AW. (1999) Singlet oxygen adducts of cholesterol: Photogeneration and reductive turnover in membrane systems. Photochem Photobiol. 70:484-489.
29. Girotti AW. (1998) Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 39:1529-1542.
30. Baker A, Kanofsky JR. (1992) Quenching of singlet oxygen by biomolecules from L1210 cells. Photochem Photobiol. 55:523-528.
07/14/08
04/17/11
05/07/13