PHOTOSENSITIZATION OF SUBCELLULAR STRUCTURES
Terje Christensen
Statens stralevern, Norwegian Radiation Protection Authority (NRPA) Boks 55, NO-1332 OSTERAS, Norway
terje.christensen@nrpa.no
When a cell responds to a photosensitizing compound combined with optical radiation, the response may be of different types. The most typical and best studied reactions are noxious to the cell; the cell may die or become genetically altered, but in some situations the cell may be stimulated to increase its synthesis of enzymes or its rate of growth for a period after the irradiation.
The magnitude and type of reaction is determined by a number of parameters, among others:
1. The light intensity.
2. The irradiation time.
3. The total dose of light, normally equal to the intensity x time.
4. The concentration of the photosensitizer.
5. The time of contact between the sensitizer and the cell.
6. The chemical structure of the sensitizer.
7. The transport of the sensitizer to the cell, and uptake in the cell.
8. The type of cell.
9. The physical and chemical characteristics of the environment,
extra- and intra-cellular.
10. The sub-cellular localization of the sensitizer.
In this module we will briefly describe the subcellular structures, and how various photosensitizers are taken up by cells and distributed among the different subcellular structures. When the compounds are localised at different sites in the cell, they will act as photosensitizers by a few basic biophysical processes, but it will be shown that the biological effects on the cell will vary substantially.
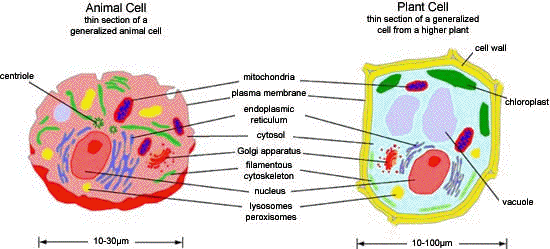
The Inside of a Cell
The cell is composed of a multitude of specialized structures, which may be grouped in:
• The outer cell membrane or plasma membrane. Plant cells and
bacteria have a cell wall composed of macromolecules
surrounding the plasma membrane.
• The cytosol, which is the liquid interior of the cell, "cell sap".
• The cytoskeleton that is composed of fibers supporting the cell
mechanically, and performing movements of the cell and its parts.
• The membrane bound organelles.
A more extensive review of cell structure can be found in some of the Suggested Readings. Alberts, et al., and Becker, et al., are both good sources.
Where Can the Photosensitizers Come From and Where Will They End Up?
When we think about photosensitization, we often assume that the effects are due to the addition of external sensitizers to a cell, but a number of sensitizers originate from the interior of the cell itself. Some amino acids and vitamins are essential to a cell's function, and will in addition have photosensitising properties. The effect of the photosensitization from natural substances coming from the organism, itself, is observed typically under extreme conditions. The synthesis of porphyrins in the cytosol and the mitochondria is exceptional, since the control points in the body can be circumvented causing a higher concentration of photosensitizing porphyrins than normal.
The group of diseases called the porphyrias can in some cases cause photosensitivity of the skin by the over-production of naturally occurring porphyrins. Water-soluble porphyrins cause increased skin fragility on light-exposed areas such as hands, neck and face. On the other hand, the less water soluble porphyrin, protoporphyrin IX, when present in the skin in the porphyric disease erythropoietic protoporphyria (EPP) will give rise to a prickling sensation in the skin, together with an erythema. The severity of the symptoms usually increases over 12-48 h after sun exposure. Chronic changes consist of circular scars, and the development of a rough orange-peel appearance of the skin.
From the above, it is clear that the properties of the photosensitizer determine its biological effects. This is even more marked when the photosensitizer is added to cells or injected. If the substance stays outside the cell, its effect is normally weak, since the local concentration of the sensitizer is low and the distance between the exterior of the cell and vital structures that can be altered by activated radiation products, such as singlet oxygen or oxygen radicals, is normally too long (see below). Once the photosensitizer is taken up, the effect increases.
Transport Mechanisms
Transport of photosensitizers from the outside of a cell to the interior can take place by two main mechanisms: passive diffusion and active uptake. A photosensitizer may diffuse through the lipid bilayer of the plasma membrane by dissolving in the lipid phase. Substances that are lipophilic will most readily dissolve in the membrane. The partition coefficient between lipid phase and water will more or less determine the efficiency of the passive diffusion-mechanism. Amphiphilic photosensitizers represent a special case, since they may readily be associated with the lipid bilayer with the apolar region of the molecule, but transport through the bilayer is hindered by polar groups.
Certain molecules may be taken up in the cells by carrier-mediated uptake, leading to a more efficient uptake than could be expected from the partition coefficient. The carriers are specialized structures that are designed to carry only molecules that fit the carrier itself. The uptake of photosensitizers bound to antibodies is an example of this mechanism. Common to both these mechanisms is that there must be a concentration gradient that drives the transport, and that no cellular energy is consumed in the process.
Active transport, on the other hand, requires a supply of energy from the cell, and can be described as pumps or as transport by vesicles that are shuttled into the cell. These vesicles are called endosomes, and the transport connects the exterior of the cell with the membrane bound compartments; lysosomes, endoplasmatic reticulum and the Golgi apparatus.
Once inside the cell, the photosensitizer will distribute between the compartments in the cell. Transport mechanisms between the compartments are of the same general types as the ones mentioned above.
Transport of the protoporphyrin analogue, hematoporphyrin and its Derivatives, is particularly well studied, because they are used in photodynamic therapy of diseases. Some uptake mechanisms are slow and some are rapid. With hematoporphyrin, the initial uptake is fairly rapid, but a saturation of the uptake is obtained only after extremely long incubation time. It has been shown that the portion of hematoporphyrin that is bound rapidly to the cell is initially present in the outer membrane, and this fraction can easily be detached from the cell. At later times the photosensitizer becomes redistributed to sites inside the cell, probably by association with membrane structures and in organelles close to the nucleus. After a longer time the sensitizer becomes more tightly bound to the cell, and its photosensitizing efficiency increases.
As a result of the uptake and distribution in a cell, the various photosensitizing substances can be found bound to more or less specific structures in the cytoplasm or the nucleus. A good example is the binding of psoralens, the plant-derived photosensitizers used together with UV-A to treat psoriasis, between the DNA strands in the double helix. Due to the close proximity to essential molecules, the psoralen molecules will become covalently bound to the strands of DNA as a result of absorption of UV-A radiation.
Redistribution of the photosensitizer in a cell may take place, induced by a change in pH in the cell, or by metabolism of the photosensitizer in the cell. Since photosensitizers are light sensitive molecules, a brief light pulse can change the physical or chemical properties of the sensitizer, and cause it to move from its original location in the cell. The skin sensitivity of patients with erythropoietic protoporphyria, mentioned above, involves a redistribution of the photosensitizer protoporphyrin IX. The protoporphyrin will detach from the primary binding sites in the red blood cells, when a dose of light reaches the capillaries, and can be transported to the skin, where the reactions that it causes are more severe.
Effects of Irradiation
Type I and II. Recall from the Basic Photosensitization Module that photosensitization reactions can be classified as either Type I or Type II. In a Type I reaction the photosensitizer transfers an electron to another molecule. In a Type II reaction, the photosensitizer transfers energy to an oxygen molecule, producing the reactive species known as singlet oxygen.
Due to the close proximity between the psoralen molecule and the DNA-strands, a Type I mechanism is likely to occur. A covalent bond between one base and the psoralen is formed if a photon is absorbed by the psoralen. Now the molecule has become glued to the DNA at one end. If a second photon is absorbed, the other end of the molecule may become attached to the opposite strand. The lesion, thus formed, prevents further cell division, because it is hard to repair by regular DNA-repair mechanisms, and the cell is likely to stop dividing. This is exactly the purpose of treatment of psoriasis, which is a disease characterized by hyper-proliferation of skin cells.
Singlet Oxygen. In real life, few things are as straightforward as the example above, and psoralen photosensitization is no exception. This traditional drug had been used for many years, it worked, and everybody was happy about the simple and logical explanation of its effect via a Type I mechanism. Eventually, however, it was shown that the psoralens are also very efficient photodynamic (Type II) sensitizers, affecting a number of targets by the production of singlet oxygen. Psoralens are similar to porphyrins in the ability to produce singlet oxygen, when the substances are present in organelles in relatively dilute solutions, and when the sensitizer is not bound in very close proximity to target molecules that may be photosensitized by a Type I mechanism.
Singlet oxygen is a reactive oxygen species, but it reacts efficiently with only particular biomolecules. The species will diffuse in the cellular environment and collide with various biomolecules until it hits a molecule with which it can react. After all the lifetime of singlet oxygen is short; on the order of 106 s (one microsecond), and during that time the reactive oxygen species is able to diffuse only about 0.1 x 106 m (one tenth of a micrometer). This distance can be compared to a typical diameter of a cell, which is more than 100 times larger. The lifetime of singlet oxygen is often higher in the lipid phase of biological membranes, and membrane damage is often seen in photosensitized reaction. But singlet oxygen can also cross interior membranes in the cell, i.e., between the cytoplasm and the nucleus.
Vesicles. If the photosensitizer is confined within a membrane bound organelle or vesicle in the cell, either dissolved in the interior or in the membrane, the production of singlet oxygen will lead to disruption of the vesicle. In the beginning of the era of research on photodynamic action it was believed that the disruption of lysosomes was the mechanism behind cell killing. The hypothesis was that the cell was digested from within by release of hydrolytic enzymes normally kept inside the lysosomes. Today it is known that this hypothesis is only partly true. Cell death by apoptosis may occur after treatment with photosensitizers and light and self degeneration by lytic enzymes is a part of the apoptotic process. It is interesting to note that uptake and targeted release of drugs and other substances by light irradiation can be performed by exploiting this well known mechanism.
Mitochondria are among the first organelles to show ultrastructural changes after photosensitization. The function of these structures is diverse, but the energy metabolism has been seen as their main function in a cell. Other functions that take place in the mitochondria, and are linked to photosensitization, are the synthesis of natural porphyrins and the control of cell death by apoptosis. It has been shown that cationic dyes can be rather selectively bound to the inner membrane of the mitochondria, and that illumination then can interfere with the respiratory functions known to take place at that site. Localization of dye to the mitochondria can also interfere with the distribution of calcium inside the cell, which in turn can induce cell death under certain circumstances.
Tubulin. Organised arrays of macromolecules like DNA and intracellular vesicles are not the only structures that can be photosensitised. Molecules in the cytoplasm can also be targets. A good example of this is the inhibition of the assembly of tubulin by photosensitization with several sensitizers. Tubulin-fibers are formed by the assembly of monomers during cell division, and are attached to the chromosomes. The fibers pull the chromosomes to the two poles of a mitotic cell, and distribute them evenly between the two daughter cells (Figure 2).
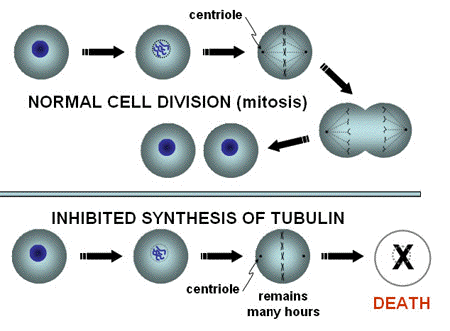
Figure 2. Tubulin-mediated inhibition of mitosis. In normal Mitosis, the chromosomes are pulled by microtubules, formed from tubulin, towards the two centrioles, marking the location where the nuclei of the two daughter cells will be formed. After that, the cell membrane is pinched off, and the chromosomes decondense in the newly formed cells. If the chromosomes are not separated by the "ropes" formed from tubulin, the normal process of mitosis will not be completed, and cell death may result.
An inhibition of the tubulin function leads to an arrest of the cells in mitosis (Figure 3), and no further cell division as long as the chromosomes are not transported to the two poles. A large number of cells have been shown to die while they are arrested in this phase of cell division. A mitotic arrest is the mechanism behind the function of mitotic inhibitors commonly used in cancer therapy (drugs of the group Vinca alkaloids).
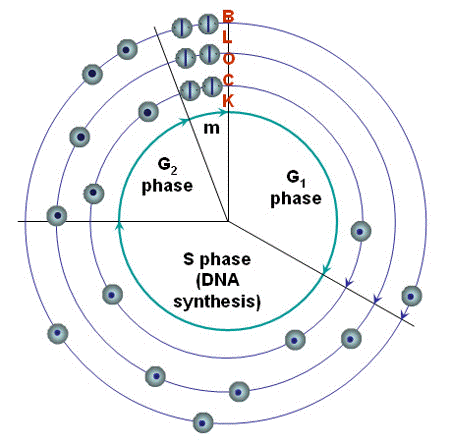
Figure 3. Cell cycle block. Blocks in the progression through the cell cycle may be formed at different points. A block at the end of G1 is known to allow the cell to repair accumulated DNA-damage if the cell has been irradiated. The figure shows a block in mitosis that has been induced by photosensitization, and illustrates how an arrest or severe inhibition in the regular progression will increase the number of cells in the phase before the block, and reduce the number of cells that can be found in the succeeding phase. It is not known if mitotic inhibition can play any role in normal cell physiology.
Photosensitization of Extracellular Structures
The outside of a cell plays an essential function by transferring signals, transporting nutrients, and attaching the cell to neighboring cells or the extracellular matrix. Specialized proteins mediate cell attachment, and these are critically important in allowing cancer cells to grow and invade other tissues, for wound healing, implantation of embryos in the uterus, etc. It has been shown that photosensitizers can modify the outside of the cell and the extracellular matrix probably by cross-linking and photooxidation of proteins. Due to reduced binding of cells to the extracellular matrix, photosensitization may reduce the risk of metastasis in cancer therapy and inhibit the blockage of coronary arteries by restenosis following angioplasty. The latter effect is important because while it is true that recent medical advances have allowed blocked or partially blocked arteries to be reopened, a process called angioplasty, these areas often close down again, often within months. Methods, such as photosensitization, that can delay this secondary blockage, called restenosis, have tremendous therapeutic potential.
Conclusion
Every structure of a cell can be photosensitized. Photosensitization can influence vital functions of the cell. Different sensitizers will be taken up differently by the cells, and cause different types of damage after irradiation with light. The relative importance of different types of damage is chiefly influenced by the distance between the bound sensitizer, and the site where the damage can take place.
Suggested Readings
Alberts, B, Johnson, A, Lewis, J, Raff, M, Roberts, K, Walter, P. Molecular Biology of the Cell, 4th edition, 2002, Garland Publishing Company.
Becker, WM, Reece, JB, Poenie, MF. The World of the Cell, 4th edition, 1999/2000, Benjamin/Cummings Publishing Co.
Boyle, RW, Dolphin, D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem Photobiol. 1996 Sep;64(3):469-85. Review.
Jori, G. Tumour photosensitizers: approaches to enhance the selectivity and efficiency of photodynamic therapy. J Photochem Photobiol B. 1996 Nov;36(2):87-93. Review.
Karger, Basel. Current Problems in Dermatology, vol 15, 1986 (Series Editor: H. Hönigsmann, Volume 15: "Therapeutic Photomedicine" Volume editors H. Hönigsmann and G. Stingl) Sections II,Therapeutic principles and III, Molecular aspects.
Peng Q, Moan J, Nesland JM. Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct Pathol. 1996 Mar-Apr;20(2):109-129. Review.
Spikes, John D., Photosensitization. In: The Science of Photobiology, Kendric C. Smith, ed. Plenum Press, New York and London, 1989, pp. 79-110.
06/04/09