THE VISUAL CYCLE
Generation of 11-cis Retinal for Photoreceptors
Rosalie K. Crouch
Storm Eye Institute, Medical University of South Carolina
167 Ashley Avenue, Charleston, South Carolina 29403
crouchrk@musc.edu
Introduction
The processing of visual information begins in the retina with the detection of light by photoreceptor cells. In humans, two specialized types of photoreceptors detect light under different conditions. Rod photoreceptors are highly sensitive and mediate vision in dim light, while cone photoreceptors function in bright light and mediate both high acuity and color vision. To detect light, both rods and cones exploit the unique properties of 11-cis retinal, a photosensitive derivative of vitamin A. The 11-cis retinal in photoreceptors is covalently bound to an opsin signaling protein to form a visual pigment molecule. In the presence of light, 11-cis retinal is isomerized to all-trans retinal, and the straightening of the polyene chain activates the opsin (Figure 1). While the formation of all-trans retinal is essential for activating photoreceptors and initiating vision, neither the opsin nor the all-trans retinal are sensitive to light, and new 11-cis retinal must be provided for photoreceptors to function continuously. Brief shortages of 11-cis retinal are an uncomfortable but common event. For example, the inability to see at night after passing an oncoming car's bright lights is partially due to the depletion of 11-cis retinal in rods. While vision recovers from transient shortages, prolonged 11-cis retinal deficits in conditions like vitamin A deficiency can eventually lead to more pronounced visual deficits. To generate enough 11-cis retinal for the normal function and survival of photoreceptors, all-trans retinal is converted back into 11-cis retinal through a series of enzymatic steps known as the visual cycle.
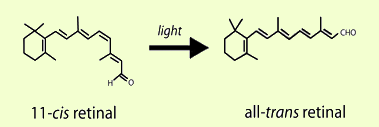
Figure 1. 11-cis Retinal is the light-sensitive component of rod and cone photoreceptors. In the first step of vision, photoreceptors are activated when light induces the isomerization of 11-cis retinal to all-trans retinal.
Visual Pigments
To generate a cellular response to light, the 11-cis retinal in photoreceptors is linked to an opsin protein capable of activating signaling pathways. Together, the 11-cis retinal and opsin protein are known as a visual pigment (Figure 2). Opsins are integral membrane proteins with seven trans-membrane helices that enclose a binding pocket for 11-cis retinal (Palczewski et al., 2000). By themselves, opsins are not photosensitive, and it is only when coupled with 11-cis retinal that the protein absorbs visible light. The absorption characteristics of different visual pigments are controlled by the interactions between 11-cis retinal (in mammals) and the opsin. For example, humans have three types of cone photoreceptors that are sensitive to red, green, and blue light. Each expresses a slightly different opsin, and the unique interactions between a particular cone opsin and 11-cis retinal result in a sensitivity to a specific wavelength (or color) of light (for review, see Ebrey and Koutalos, 2001). Despite absorbing different wavelengths of light, the general relationship between the opsin and 11-cis retinal is the same in all visual pigments. In the dark, 11-cis retinal binds the opsin as an inverse agonist and holds the opsin in an inactive conformation. When light strikes the visual pigment, the isomerization of 11-cis retinal to all-trans retinal in the binding pocket pushes the opsin into an active conformation and initiates phototransduction. While the newly formed all-trans retinal is needed to activate the opsin, it lacks photosensitivity, and the opsin must release all-trans retinal and bind new 11-cis retinal to continue detecting light.
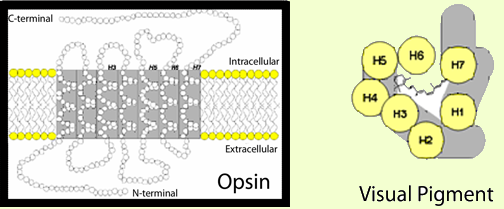
Figure 2. Visual pigments are responsible for initiating a photoreceptor's response to light. Each visual pigment molecule consists of an 11-cis retinal chromophore covalently bound to an opsin G-protein coupled receptor. 11-cis Retinal is the light-sensitive component of visual pigments. The opsin constitutes the signaling component. The framed image depicts a two-dimensional representation of an opsin protein with its seven trans-membrane alpha-helices. Helices 3, 5, 6, and 7 are thought to line the opsin binding pocket. In the overhead depiction of a visual pigment (to the right), 11-cis retinal is shown in the binding pocket formed by the opsin helices (H1-H7).
Anatomy Of The Visual Cycle
The anatomical relationship between the photoreceptors and retinal pigment epithelium (RPE) is critical to the visual cycle. A view of the retina in cross-section reveals that the photoreceptors are paradoxically located in the deepest retina layer, and the light-sensitive outer segments are the farthest retina layer from light entering the pupil (Figure 3). Although this arrangement requires incoming light to pass through each retina layer before reaching the photoreceptors, it positions the outer segments in close proximity to the specialized monolayer of RPE cells. While not a part of the neural retina, the RPE is essential for the normal function and survival of photoreceptors. Among its many contributions to the photoreceptors, the RPE is the principle site for 11-cis retinal regeneration in the visual cycle.
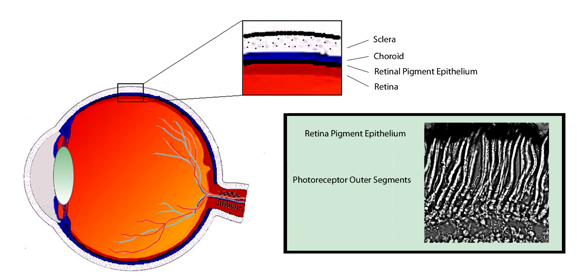
Figure 3. Photoreceptors rely on the retinal pigment epithelium (RPE) for numerous metabolic functions, including the visual cycle. In cross section, photoreceptors are located in the back of the retina in close association with the RPE. The colored insert shows a micrograph of Xenopus laevis photoreceptors, and demonstrates the close anatomical relationship between the photoreceptor outer segments and the RPE.
The Classical Visual Cycle
The classical visual cycle regenerates 11-cis retinal through a series of steps involving specialized enzymes and retinoid binding proteins, and the importance of each step is underscored by the fact that each has been identified as sources of visual impairment or blindness in humans (Travis et al., 2007). Our understanding of the visual cycle is largely based on studies from rod photoreceptors. Cones are believed to rely upon the same system, but are also thought to have privileged access to alternate visual cycle pathways in the inner retina. With growing evidence of a cone-specific pathway (for review, see Wolf, 2004), the cycle occurring between the photoreceptors and RPE is increasingly referred to as the classical visual cycle (Figure 4).
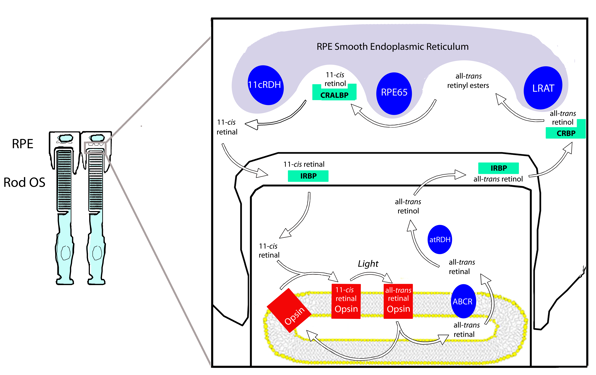
Figure 4. The classical visual cycle involves the cycling of retinoids between the rod outer segments (OS) and the RPE. The visual cycle begins in the outer segment with all-trans retinal's release from the opsin. After reduction to all-trans retinol, the photoproducts cross the sub-retinal space and enter the RPE. Here, 11-cis retinal is regenerated in three enzymatic steps and returned to the photoreceptors. IRBP is thought to transport retinoids through the sub-retinal space.
The classical visual cycle begins in the rod outer segment with the absorption of a photon by a visual pigment molecule. Rod outer segments contain stacks of membranous discs made of a lipid bi-layer. all-trans Retinal is released from the activated opsin into inner leaflet of the disc bi-layer and is believed to complex with phosphatidylethanolamine. The resulting N-retinylidine-phosphatidylethanolamine is transported to the cytoplasmic disc surface by the retina specific ATP binding cassette transporter (ABCR), and released into the cytoplasm as all-trans retinal (Liu et al., 2000). Once in the cytoplasm, all-trans retinal is reduced to all-trans-retinol (Vitamin A) by all-trans retinol dehydrogenase (at-RDH) in an NADPH-dependent reaction (Haeseleer et al., 1998). all-trans Retinol then exits the photoreceptor, crosses the sub-retinal space bound to the interphotoreceptor retinoid binding protein (IRBP), and enters the RPE (Bunt-Milam and Saari, 1983; Okajima et al., 1994; Ala-Laurila et al., 2006; Wu et al., 2007).
In the RPE, at least three enzymes associated with the smooth endoplasmic reticulum convert all-trans retinol to 11-cis retinal. After entering an RPE cell, all-trans retinol is transferred to the cellular retinoid biding protein (CRBP) (Saari et al., 1982) and delivered to the first visual cycle enzyme in the RPE, lecithin retinol acyl transferase (LRAT) (Saari and Bredberg, 1989). LRAT links all-trans retinol to phosphatidyl choline in the membrane to generate all-trans retinyl esters. Additionally, all-trans retinol from systemic circulation can enter the visual cycle through the basal surface of RPE cells for esterification by LRAT. The esters generated by LRAT are the primary storage form of retinoids in the eye, and their accumulation is thought to be an important force driving subsequent reactions in the visual cycle. More importantly, they serve as the substrate for the next step of the visual cycle and are required for 11-cis retinal regeneration (Moiseyev et al., 2003).
The next step of the visual cycle involves the simultaneous hydrolysis and isomerization of all-trans retinyl esters to yield 11-cis retinol. The coupling of isomerization and hydrolysis is facilitated by a single enzyme, generically termed the isomerohydrolase, and thought to be the RPE65 protein (Deigner et al., 1989; Redmond et al., 1998; Mata et al., 2004; Jin et al., 2005). Indeed, RPE65 is essential for the regeneration of 11-cis retinoids, and there is no isomerohydrolase activity in its absence (Redmond et al., 2005). 11-cis Retinol from the isomerhydrolase reaction binds the cellular retinaldyhyde binding protein (CRALBP), a retinoid binding protein with high affinity for 11-cis retinoids (Saari et al., 2001).
CRALBP delivers the 11-cis retinol to 11-cis retinol dehydrogenase (11-cis RDH) for the third and final enzymatic step in the RPE. 11-cis RDH oxidizes 11-cis retinol to 11-cis retinal using NAD as a cofactor (Driessen et al., 1995; Simon et al., 1995), and newly generated 11-cis retinal crosses the sub-retinal space and re-enters the photoreceptors. Again, IRBP is proposed to facilitate this transport and protect 11-cis retinal from isomerization en route to the photoreceptors (Jones et al., 1989; Crouch et al., 1992; Ala-Laurila et al., 2006; Wu et al., 2007). After entering the outer segment, the newly generated 11-cis retinal can bind with opsin and regenerate functional visual pigment to complete the cycle.
The Cone Visual Cycle
The classical visual cycle associated with the outer segment and RPE is well-studied in rods and thought to apply to cones, but cones are theorized to have unique visual cycle pathways. Cones, which are responsible for the bulk of human vision, are specialized for functioning in daytime vision where constant light increases the demand for 11-cis retinal. While the classical visual cycle involves the photoreceptors and RPE, the cone visual cycle is believed to involve the cone photoreceptors and the Müller glia cells of the inner retina. Central to this theory is the unique ability of cones to regenerate 11-cis retinal from 11-cis retinol supplied to the inner segment (Jones et al., 1989). In the proposed cone visual cycle, all-trans retinol generated in photoreceptors is transported to Müller cells and isomerized to 11-cis retinol by an unidentified isomerase. 11-cis Retinol from Müller cells then enters the inner segments of cone photoreceptors and is oxidized to 11-cis retinal for visual pigment formation. Interestingly, cone inner segments are in close proximity to the apical microvilli of Müller cells, and these microvilli contain CRALBP, a retinoid binding protein with a high affinity for 11-cis retinoids (Bunt-Milam and Saari, 1983). Müller cells have the ability to generate 11-cis retinol from all-trans retinol (Das et al., 1992). Furthermore, IRBP, the transporter discussed in the classical visual cycle, co-localizes with the microvilli, is a major carrier of 11-cis retinol (Saari et al., 1985), and is found in high concentrations around cone photoreceptors (Carter-Dawson and Burroughs, 1992a, b; Gonzalez-Fernandez, 2003). Although a functional relationship between IRBP, Müller cells, and cone inner segments in a cone-specific pathway has not been proven, it would explain the ability of cones to efficiently regenerate visual pigment in constant light conditions.
Summary
The photoisomerization of 11-cis retinal to all-trans retinal in photoreceptors is the first step in vision, but the continued function of photoreceptors requires that all-trans retinal be converted back into 11-cis retinal via the visual cycle. In the visual cycle, all-trans retinal is released from the activated opsin and reduced to all-trans retinol in the photoreceptor outer segment. IRBP is believed to facilitate the transport of all-trans retinol from the outer segments to the RPE, where all-trans retinol is used to generate all-trans retinyl-esters with LRAT. These esters serve as the substrate for an isomerohydrolase reaction, likely catalyzed by RPE65, which generates 11-cis retinol. 11-cis RDH then oxidizes 11-cis retinol into 11-cis retinal. To complete the visual cycle, newly generated 11-cis retinal is transported back across the sub-retinal space to the photoreceptors to regenerate photosensitive visual pigment.
References
Ala-Laurila P, Kolesnikov AV, Crouch RK, Tsina E, Shukolyukov SA, Govardovskii VI, Koutalos Y, Wiggert B, Estevez ME, Cornwall MC (2006) Visual cycle: Dependence of retinol production and removal on photoproduct decay and cell morphology. J Gen Physiol 128:153-169.
Bunt-Milam AH, Saari JC (1983) Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol 97:703-712.
Carter-Dawson L, Burroughs M (1992a) Interphotoreceptor retinoid-binding protein in the Golgi apparatus of monkey foveal cones. Electron microscopic immunocytochemical localization. Invest Ophthalmol Vis Sci 33:1589-1594.
Carter-Dawson L, Burroughs M (1992b) Interphotoreceptor retinoid-binding protein in the cone matrix sheath. Electron microscopic immunocytochemical localization. Invest Ophthalmol Vis Sci 33:1584-1588.
Crouch RK, Hazard ES, Lind T, Wiggert B, Chader G, Corson DW (1992) Interphotoreceptor retinoid-binding protein and alpha-tocopherol preserve the isomeric and oxidation state of retinol. Photochem Photobiol 56:251-255.
Das SR, Bhardwaj N, Kjeldbye H, Gouras P (1992) Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J 285 ( Pt 3):907-913.
Deigner PS, Law WC, Canada FJ, Rando RR (1989) Membranes as the energy source in the endergonic transformation of vitamin A to 11-cis-retinol. Science 244:968-971.
Driessen CA, Janssen BP, Winkens HJ, van Vugt AH, de Leeuw TL, Janssen JJ (1995) Cloning and expression of a cDNA encoding bovine retinal pigment epithelial 11-cis retinol dehydrogenase. Invest Ophthalmol Vis Sci 36:1988-1996.
Ebrey T, Koutalos Y (2001) Vertebrate photoreceptors. Prog Retin Eye Res 20:49-94.
Gonzalez-Fernandez F (2003) Interphotoreceptor retinoid-binding protein--an old gene for new eyes. Vision Res 43:3021-3036.
Haeseleer F, Huang J, Lebioda L, Saari JC, Palczewski K (1998) Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem 273:21790-21799.
Jin M, Li S, Moghrabi WN, Sun H, Travis GH (2005) Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell 122:449-459.
Jones GJ, Crouch RK, Wiggert B, Cornwall MC, Chader GJ (1989) Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci USA 86:9606-9610.
Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR (2000) The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem 275:29354-29360.
Mata NL, Moghrabi WN, Lee JS, Bui TV, Radu RA, Horwitz J, Travis GH (2004) Rpe65 is a retinyl ester binding protein that presents insoluble substrate to the isomerase in retinal pigment epithelial cells. J Biol Chem 279:635-643.
Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr., Redmond TM, Ma JX (2003) Retinyl esters are the substrate for isomerohydrolase. Biochemistry 42:2229-2238.
Okajima TI, Wiggert B, Chader GJ, Pepperberg DR (1994) Retinoid processing in retinal pigment epithelium of toad (Bufo marinus). J Biol Chem 269:21983-21989.
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M (2000) Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289:739-745.
Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K (1998) Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet 20:344-351.
Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S (2005) Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A 102:13658-13663.
Saari JC, Bredberg DL (1989) Lecithin retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem 264:8636-8640.
Saari JC, Bredberg L, Garwin GG (1982) Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J Biol Chem 257:13329-13333.
Saari JC, Teller DC, Crabb JW, Bredberg L (1985) Properties of an interphotoreceptor retinoid-binding protein from bovine retina. J Biol Chem 260:195-201.
Saari JC, Nawrot M, Kennedy BN, Garwin GG, Hurley JB, Huang J, Possin DE, Crabb JW (2001) Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron 29:739-748.
Simon A, Hellman U, Wernstedt C, Eriksson U (1995) The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem 270:1107-1112.
Travis GH, Golczak M, Moise AR, Palczewski K (2007) Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol 47:469-512.
Wolf G (2004) The visual cycle of the cone photoreceptors of the retina. Nutr Rev 62:283-286.
Wu Q, Blakeley LR, Cornwall MC, Crouch RK, Wiggert BN, Koutalos Y (2007) Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry 46:8669-8679.
02/16/09