PHOTOBIOLOGY of the UVEAL TRACT
Dan-Ning Hu
The New York Eye and Ear Infirmary, Tissue Culture Center
310 East 14th Street, New York, NY 10003
hu2095@yahoo.com
Introduction
The uveal tract is a layer of tissue located between the outer layer (cornea and sclera) and the inner layer (the retina) of the eye. The front portion (anterior) of the uveal tract contains the iris, and the back portion (posterior) of the uveal tract contains the choroid and the stroma of the ciliary body. The iris is exposed to both visible light and UV radiation, and the choroid and ciliary body are mostly sheltered from visible light and UV radiation. The uveal tract is deeply pigmented so that the iris also acts as a light screen to eliminate excessive light entering the lens and retina (Figure 1).
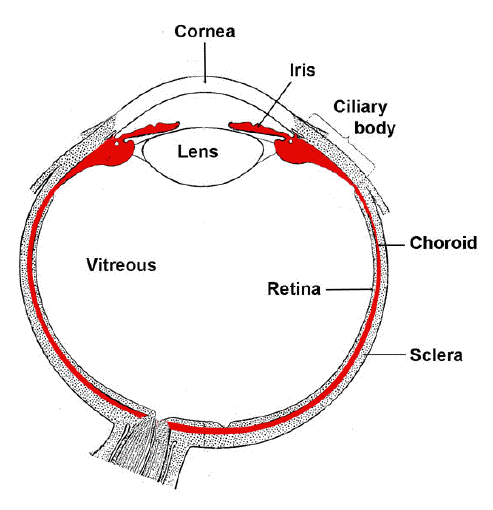
Figure 1. Structure of the eye with uveal tract labeled red. The uvea can be divided into three parts, the iris, the ciliary body, and the choroids.
There are two different types of pigmented cells in the uveal tract, the melanocytes and pigment epithelial cells. The melanocytes located in the iris are constantly exposed to UV radiation, and this leads to the malignant transformation of these cells to form a specific type of malignant tumor, the uveal melanoma. As the choroidal and ciliary melanocytes are shielded from UV radiation, only iris melanoma is thought to be triggered by the detrimental effects of UV radiation
[Hu, 2005b, 2008].
Structure And Function Of The Uveal Tract
The wall of the human eye consists of three distinct concentric layers, the outer layer (the transparent cornea and the opaque white sclera), the middle layer (the uveal tract, or uvea), and the inner layer (the retina). The uvea is composed of three parts: the iris anteriorly, an intermediate ciliary body, and the choroid posteriorly. The uveal tract is a highly vascularized connective tissue, and contains various cell types
[Hu, 2002].
The iris. The iris is the anterior part of the uvea, and forms a diaphragm over the lens. There is a hole in the center of the iris, which is known as the pupil. Pupil size is controlled by the light intensity entering the eye. It contracts under intensive light irradiation, and expands under low lighting, thus allowing more or less light to enter the lens and the posterior portion of the eye.
The iris has three different layers. The first layer consists of melanocytes and fibroblasts, which cover the anterior part of the iris. The second part contains the main mass of the iris (iris stroma). The iris stroma is a layer rich in blood vessels embedded in loose connective tissues, which contain various cells, such as fibroblasts and melanocytes. The third layer consists of two layers of pigment cells (iris pigment epithelium). The anterior iris pigment epithelium is a layer of smooth muscle cells that control the size of the pupil. The posterior iris pigment epithelium is a layer of densely pigmented cells, which covers the posterior layer of the iris [Jakobiec, 1982].
The color of the human iris varies from blue to dark brown, and the difference of the iris color is mainly determined by the type and amount of melanin in the melanocytes [Eagle, 1988; Imesch, 1996; Hu, 1995; Prota, 1998; Wakamatsu, 2008; Wilkerson, 1996]. The melanin in the eye is either eumelanin, which is black to brown, or pheomelanin, which is yellow to reddish [Wakamatsu, 2008]. Iridal melanocytes in a dark colored iris (brown and dark brown colored irides) contain a high ratio of eumelanin/pheomelanin, and more melanin than melanocytes from light colored irides [Prota, 1998; Wakamatsu, 2008]. In the light colored iris (blue, yellow brown and green colored irides), iridal melanocytes contain less melanin, especially eumelanin. Iris pigment epithelium contain mainly eumelanin. There is no difference in melanin content in the iris pigment epithelium in normal irides of dark or light color. In albinism, both melanocytes and iris pigment epithelium lack pigment, and the iris becomes pink in color [Hu, 2008; Nordlund, 1998].
The iris has various functions, which include a light screening effect that can eliminate stray light rays. The size of the pupil responds to the light intensity entering the eye, and controls the amount of light entering the lens and the posterior segment of the eye [Jakobiec, 1982].
The ciliary body. The ciliary body is located between the iris and the choroid, and is attached to the outer layer of the eye (the sclera). It extends for approximately 5-6 mm from the root of the iris to the beginning of the choroid. The structure of the ciliary body is similar to the iris, which consists of the stroma and the ciliary epithelium. The ciliary epithelium covers the inner surface of the ciliary body, and consists of two different layers of cells, the pigmented and non-pigmented ciliary epithelial cells [Jakobiec, 1982].
The main function of the ciliary body is the production of the aqueous humor which fills the space in front of and behind the iris. Contraction of ciliary muscle controls the shape of the lens through the zonule, which is attached to the ciliary body and the lens [Jakobiec, 1982].
The choroid. The choroid is the posterior part of the uvea, and is located between the outer layer (sclera) and inner layer (retina) of the eye. The choroid is a layer of connective tissue, which consists of large amount of blood vessels. In contrast to the iris and ciliary body, the pigment epithelium cells in this region (retinal pigment epithelium) are classified as a part of the retina rather than the uvea. The main function of the choroid is to support and nourish the retina [Jakobiec, 1982].
Photobiology Of The Iris
Accessibility of the iris to light radiation. The iris is covered by the cornea and the aqueous humor. Most visible light (400 - 750 nm) can pass through the cornea and the aqueous humor, and reaches the anterior surface of the iris. Virtually all UV radiation below 295 nm (UV-C and part of UV-B) is blocked by the cornea, and does not reach the iris [Boettner, 1962; Lerman, 1980; Hu, 2008; Roberts, 2000, 2001].
The transmission of UV radiation at 290 - 400 nm (UV-A and UV-B) through the cornea and the aqueous humor can be expressed by two different parameters: the direct transmittance and the total transmittance. When UV radiation passes through a medium, part of it can pass directly through the medium, part of it is absorbed or reflected by the medium, and the remainder can be scattered by the medium. The direct transmission is the UV radiation that passes directly through the medium, and the total transmittance of radiation combines both the direct transmission and the scattered radiation. The direct and total transmittance of UV radiation that pass through the cornea and aqueous humor are 20% and 50% at 320 nm (UV-B); 32% - 42% and 55 - 70% at 350 - 400 nm (UV-A), respectively [Boettner, 1962]. The transmission of UV-A and UV-B through the cornea and the aqueous humor is age dependent. The total transmission of UV radiation through the cornea and the aqueous humor by 8, 24 and 80 year old individuals is 20%, 19% and 17% at 320 nm; 80%, 50% and 32% at 350 nm and 98%, 60% and 40% at 400 nm, respectively [Lerman 1980]. Therefore, the light radiation that reaches the anterior surface of the iris is approximately less than 5% of all UV-C, 5 - 20% of UV-B, 20 -60% of UV-A and 60 - 90% of visible light in adult human being [Boettner, 1962; Lerman, 1980].
Transmission of light radiation through the iris. The iris is a tissue containing various pigments, mainly the melanin and hemoglobin. Therefore the iris can absorb and scatter a larger proportion of visible light and UV radiation entering its surface, and functions as a light screen [Hong, 2006; Hu, 2008].
Photobiology of melanin. Melanin has a protective effect on the cells and tissues, which consists of the photoscreening effect and the biophysical/biochemical effect [Hong, 2006; Hu, 2008; Wang, 2006]. The effect of uveal melanin is related to its location. In the anterior segment (the iris), which is exposed to sunlight and UV radiation, the photoscreening effect dominates. Melanin absorbs near-infrared, visible light, and UV radiation, with absorption increasing at the shorter wavelengths. In the iris, the pigment epithelium and the melanocytes absorb and block visible light and UV radiation, thus, they possess photoprotective effects [Hong, 2006; Hu, 2002, 2008; Sarna, 1992, 1998].
There are two chemical forms of melanin, eumelanin and pheomelanin. Several studies have compared the reactivity of eumelanin and pheomelanin, and they have found that both melanins act as a free radical scavenger and inhibit UV-induced lipid peroxidation. However, when pheomelanin is complexed with Fe (III), it stimulates UV-induced lipid peroxidation, whereas eumelanin does not [Krol, 1998]. The antioxidant property of melanin is related to the type of melanin: the greater the ratio of eumelanin to pheomelanin, the better the antioxidant capacity of the melanin [Chedekel, 1980]. The higher the melanin and eumelanin content of cultured melanocytes the better the rate survival after UVB irradiation [de Leeuw, 2001]. UV irradiation of pheomelanin may also generate reactive oxygen species whereas eumelanin tends to be stable [Takeuchi, 2004]. Recently, Samokhvalov (2005) found that pheomelanosmes are more prooxidant than eummelanosomes and this difference is attributed to the pigment content of the melanosome, namely whether it contains eumelanin and pheomelanin.
Eye Diseases Related To Light Irradiation On The Iris
Iris melanoma. Melanoma is caused by the malignant transformation of melanocytes. Studies on cutaneous melanoma have demonstrated that the occurrence of this disorder is related to the detrimental effects caused by UV radiation, which cause gene mutations that lead to the occurrence of melanoma. Oxidative stress also plays a role in the malignant transformation of melanocytes [Nordlund, 1998].
Melanoma occurring in the uveal tract is uveal melanoma. A population based study on the relationship of racial/ethnic groups and incidence of uveal melanoma found that the incidence of uveal melanoma is highest in non-Hispanic whites, followed by Hispanics, Asians and blacks, indicating that the lighter colored eyes have a higher risk of uveal melanoma [Hu., 2005a]. A meta analysis of the relationship between iris color and the incidence of uveal melanoma based on 10 studies (1732 cases) revealed that light colored irides (blue or grey) are a statistically significant risk factor for the development of uveal melanoma [Weis, 2006].
Iris melanocytes are mainly located in the anterior surface, and are exposed to sun radiation. Iris melanoma tends to occur in the inferior sector of the iris, where exposure to sunlight is the greatest [Shields, 1992], indicating that the occurrence of iris melanoma is related to exposure to UV radiation. The lower incidence of iris melanoma in dark colored eyes is possibly related to the photoscreening effect provided by a greater amount of iridal melanin [Hu, 2008].
Age-related macular degeneration. Age-related macular degeneration is a common ocular disease, which is the major cause of blindness among the elderly in developed countries. Oxidative stress, including the light induced production of various reactive oxidative species in the presence of light sensitive factors, may play an important role in the occurrence of age-related macular degeneration [Roberts, 2001; Beatty, 2000; Winkler, 1999]. Age-related macular degeneration is more prevalent in white populations than in darkly pigmented races, indicating that melanin may be protective against the development of age-related macular degeneration [Age-Related Eye Disease Study Research Group, 2000; Frank, 2000; Friedman, 1999; Klein, 1995, 2003, 2006; Sandberg, 1994; Weiter, 1985]. The protective effects of melanin on the occurrence of age-related macular degeneration in eyes with dark-colored irides are most probably related to the biophysical/biochemical effect of melanin in the choroid [Hong, 2006; Hu, 2008). However, the more effective light-screening effects of dark colored irides, which can reduce the light energy entering the posterior segment of the eye, cannot be ruled out.
Aniridia and albinism. Aniridia is the absence of iris. This can be caused by genetic factors or environmental factors (trauma). Albinism is a congenital defect, and is characterized by the absence of melanin in the skin and eye. Both aniridia and albinism cause a significant increase of light energy entering the retina, and result in photophobia (a symptom of excessive sensitivity to light, and the aversion to sunlight or well-lit places) [Yanoff, 1999]. The causal relationship between the increase of light entering the eye in these two diseases, and early appearance of cataracts or macular degeneration, has not as yet been thoroughly investigated.
Photobiology Of The Choroid And Ciliary Body
Accessibility of the choroid and ciliary body to light radiation. The choroid is covered externally by thick and non-transparent sclera and internally by the lens, the vitreous humor, the retina, and more importantly, by the densely pigmented retinal pigment epithelium. Neither visible light nor UV radiation can reach the choroid [Boettner, 1962; Hu, 2008; Lerman, 1980], although there is a window of UV radiation at 320 nm that reaches the posterior section of the eye in the first three years of life [Roberts, 2001].
The ciliary body is covered by the lens and aqueous humor internally and externally by the sclera, only the light that passes through the lens can enter the inner surface of the ciliary body. Virtually, no visible light or UV radiation can pass through the ciliary pigment epithelium and enter the stroma of the ciliary body [Boettner, 1962; Hu, 2008; Lerman, 1980].
Diseases of the choroid and ciliary body related to light radiation (choroidal and ciliary body melanoma). Uveal melanoma is the most common intraocular malignant tumor in human adults in the western countries. Most uveal melanomas occur in the choroid and ciliary body (posterior melanoma). Epidemiology studies indicate that the risk of uveal melanoma is correlated with iris color, eyes with light colored irides have a higher incidence of uveal melanoma as compared to those with dark colored irides [Holly, 1990; Hu, 2005a; Pane, 2000; Vajdic, 2001; Weis, 2006].
Controversy exists over the causal relationship between UV radiation and the occurrence of posterior melanoma, and over the explanation of the increased risk of melanoma in light colored irides. Previously, it has been assumed that malignant changes in the melanocytes can be caused by UV radiation. Choroid and ciliary body melanocytes in eyes with light colored irides are exposed to a greater amount of UV radiation as compared to eyes with dark colored irides. Therefore, the incidence of choroidal and ciliary body melanomas is higher in eyes with light colored irides.
The relationship between sun and UV radiation and the occurrence of melanoma can be determined by two parameters, the incidence of melanoma at different times and locations. If sun and UV radiation play a role in the occurrence of uveal melanoma, as in cutaneous melanoma, the incidence of uveal melanoma should have an upward trend during recent years, and a trend in increasing incidence with decreasing latitude (increasing sun radiation) [Hu, 2002, 2008; Popescu, 1990; Tuomaala, 2002; Yu, 2003, 2006].
These two factors have been studied previously in ocular melanomas, and conflicting results were obtained. Analysis of previous studies found that there were several factors leading to these conflicting results.
(1) Ocular melanomas include at least two different melanomas, conjunctival melanoma which is exposed to sunlight and uveal melanomas, which are mainly not exposed to sunlight (iris melanoma is located in the area exposed to sunlight, but it only accounted for less than 10% of uveal melanoma, most of uveal melanomas are located in the choroid and ciliary body, and are not exposed to sun and UV radiation). Several studies mixed these two different types of melanoma [Scotto, 1976; Swerdlow, 1983]. This may neutralize any possible effects, and make the results equivocal [Yu, 2006].
(2) Many studies on the relationship between sun exposure and occurrence of uveal melanoma used case-control study methodology [Gallagher, 1985; Holly, 1990; Pane, 2000; Tucker, 1985; Vajdic, 2001]. Case-control studies have inherent limitations, because they rely on questionnaires about lifestyle that occurred many years before the onset of disease. Sun exposure is difficult to estimate accurately using this method. Recall bias also confounds the data, because one is prone to reflect on the cause of their disease more than one not affected by the disease. Answers may be influenced by unproven hypotheses (e.g., UV light may cause uveal melanoma). Furthermore, using of inappropriate subjects as controls also may lead to inaccurate results [Yu, 2006]
(3) Population based study is a more reliable method than case-controlled study. However, uveal melanoma has a relatively low incidence (only 0.6 cases per one million populations per year). [Hu, 2005a; Singh, 2003]. Such a low incidence may easily produce unstable or nonsignificant results [Yu, 2006].
Recently, several studies were performed in a large cancer registry population (approximately 14% of the United States populations), age-adjusted incidence rates were calculated separately for melanomas of the conjunctiva and uvea in non-Hispanic whites only [Yu, 2003, 2006]. These studies showed that the incidence of conjunctival melanoma has an upward trend during recent years, and a trend in increasing incidence with decreasing latitude (increasing sun radiation). On the contrary, the incidence of uveal melanoma does not have these trends [Bergman, 2002; Yu, 2006]. This is consistent with the results of photobiological studies (the choroid and ciliary melanocytes are not exposed to sun and UV radiation). Furthermore, statistical analysis showed that the incidence of uveal melanoma has a slight but significant decrease in recent years, and in areas with more sun radiation (decreasing latitude) [Bergman, 2002; Yu, 2006].
These unpredicted results indicate that sun radiation may decrease the incidence of uveal melanoma. This result may be relevant to the dual effects of UV radiation on the occurrence of malignant tumors determined by their accessibility of sun radiation. Sun radiation reduces the risk and/or mortality of various systemic malignant tumors that are not exposed to the sunlight, e.g., non-Hodgkin lymphoma, and prostate, breast, colon and ovarian cancers. UV radiation increases vitamin D synthesis in the skin, which converts to 1,25-dehydroxyvitamin D3, and inhibits growth and induces apoptosis of various malignant tumor cells in vitro and in experimental animal models [Hu, 2008; Osborne, 2002; Robinson, 2005; Yu, 2006]. Therefore, sunlight has dual effects on malignant tumors: a direct mutagenic effect on tissues exposed to the sunlight, and an indirect protective effect on tissues not exposed to sunlight. Cutaneous and conjunctival melanocytes are mainly exposed to sun radiation, therefore, the direct effect of UV radiation predominates and causes an increase of incidence. Uveal melanocytes, mainly the choroidal and ciliary body melanocytes, are not exposed to sun radiation. No direct effect of sun radiation would be expected to occur in these locations. Therefore, the indirect protective effect of sun radiation causes a decrease of uveal melanoma [Hu, 2008; Yu, 2006].
The high risk of choroidal and ciliary body melanomas in eyes with light colored irides can be explained by the antioxidant effect of melanin. Oxidant stress can cause gene mutations and leads to the malignant transformation of melanocytes. Antioxidant and free radical scavenger effects of uveal melanin can protect melanocytes from oxidative stress, and reduce the occurrence of melanoma. Melanocytes in dark-colored eyes have a high quantity of melanin, which is more protective than that in light-colored eyes, therefore, the incidence of uveal melanoma is markedly less than that in the light colored eye [Hu, 2008].
Conclusion
The iris absorbs and scatters a large proportion of visible light and UV radiation entering its surface, and functions as a light screen. Only light passing through the pupil can enter the ocular tissues behind the iris plane. The iris melanocytes are exposed to sun light (including UV radiation), which may play a role in the occurrence of iris melanoma. Melanocytes located in the ciliary body and choroid are protected by the lens, and the pigment epithelium. Therefore, UV radiation cannot enter these cells, and does not play a role in the occurrence of melanoma in these locations.
References
Age-Related Eye Disease Study Research Group. (2000) Risk factors associated with age-related macular degeneration. a case-control study in the age-related eye disease study: age-related eye disease study report number 3. Ophthalmology 107:2224-32.
Beatty S, Koh H, Phil M, Henson D, Boulton M. (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 45:115-34
Bergman L, Seregard S, Nilsson B, Ringborg U; Lundell G; Ragnarsson-Olding. (2002) Incidence of uveal melanoma in Sweden from 1960-1998. Invest Ophthalmol Vis Sci. 43;2579-83.
Boettner EA, Wolter JR. (1962) Transmission of the ocular media. Invest Ophthalmol. 1:776.
Chedekel MR, Agin PP, Sayre RM. (1980) Photochemistry of pheomelanin: action spectrum for superoxide production. Photochem Photobiol. 31:553-5.
Eagle RC Jr. (1988) Iris pigmentation and pigmented lesions: an ultrastructural study. Trans Am Ophthalmol Soc. 84:581-687.
Frank RN, Puklin JE, Stock C, Canter LA. (2000) Race, iris color, and age-related macular degeneration. Trans Am Ophthalmol Soc. 98:109-15.
Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. (1999) Racial differences in the prevalence of age-related macular degeneration: the Baltimore eye survey. Ophthalmology 106:1049-55.
Gallagher RP, Elwood J M, Rootman J, Spinelli JJ, Hill GB, Therlfall WJ, Birdsell JM. (1985) Risk factors for ocular melanoma: Western Canada Melanoma Study. J Natl Cancer Inst. 74:775-8.
Holly EA, Aston DA, Char DH, Kristiansen JJ, Ahn DK. (1990) Uveal melanoma in relation to ultraviolet light exposure and host factors. Cancer Res. 50:5773-7.
Hong L, Simon JD, Sarna T. (2006) Melanin structure and the potential functions of uveal melanosomes. Pigment Cell Res. 19:465-6.
Hu DN, McCormick SA, Orlow SJ, Rosemblat S, Lin AY, Wo K. (1995) Melanogenesis in cultured human uveal melanocytes. Invest Ophthalmol Vis Sci. 36:931-8.
Hu DN, Savage H, Roberts JE. (2002) Uveal melanocytes, ocular pigment epithelium and Mueller cells in culture: in vitro toxicology. Inter J Toxicol. 21:465-72.
Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. (2005a) Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol. 140:612-7.
Hu DN. (2005b) Photobiology of ocular melanocytes and melanoma. Photochem Photobiol. 81:506-9.
Hu DN, Simon JD, Sarna T. (2008) Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol. 84:639-44.
Imesch PD, Bindley CD, Khademian Z, Ladd B, Gangnon R, Albert DM, Wallow IHL. (1996) Melanocytes and iris color: electron microscope findings. Arch Ophthalmol. 114:443-7.
Jakobiec FA. (1982) Ocular anatomy, embryology and teratology. Harper & Row, Pub., Philadelphia.
Klein R, Rowland ML, Harris MI. (1995) Racial/ethnic differences in age-related maculopathy third national health and nutrition examination survey. Ophthalmology 102:371-81.
Klein R, Klein BE, Marino EK, Kuller LH, Furberg C, Burke GL, Hubbard LD. (2003) Early age-related maculopathy in the cardiovascular health study. Ophthalmology 110:25-33.
Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, Burke G, Saad MF, and Jacobs DR Jr. (2006) Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology 113:373-80.
Krol ES, Liebler DC. (1998) Photoprotective actions of natural and synthetic melanins. Chem Res Toxicol. 11:1434-40.
De Leeuw SM, Smit NP, Van Veldhoven M, Pennings EM, Pavel S, Simons JW, Schothorst AA. (2001) Melanin content of cultured human melanocytes and UV-induced cytotoxicity. J Photochem Photobiol B. 61:106-13.
Lerman S. (1980) Radiant energy and the eye. Macmillan Pub., New York.
Nordlund JJ, Boissy RE, Hearing VJ, King RA, Ortonne J. (1998) The pigmentary system: Physiology and pathophysiology. Oxford University Press, New York.
Osborne JE, Hutchinson PE. (2002) Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 147:197-213.
Pane AR, Hirst LW. (2000) Ultraviolet light exposure as a risk factor for ocular melanoma in Queensland, Australia. Ophthalmic Epidemiol. 7:159-67.
Popescu NA, Beard CM, Treacy PJ, Winkelmann RK, O'Brien PC, Kurland LT. (1990) Cutaneous malignant melanoma in Rochester, Minnesota: trends in incidence and survivorship, 1950 through 1985. Mayo Clin Proc. 65:1293-302.
Prota G, Hu DN, Vincensi MR, McCormick SA, Napolitano A. (1998) Characterization of melanins in human irides and cultured uveal melanocytes from eyes of different colors. Exp Eye Res. 67:293-9.
Roberts, JE. (2000) Light and immunomodulation. NY Acad Sci. 917:435-445.
Roberts JE. (2001) Ocular Phototoxicity. J Photochem Photobiol B. 64:136-43.
Robinson J K. (2005) Sun exposure, sun protection, and vitamin D. JAMA. 294:1541-3. Samokhvalov A, Hong L, Liu Y, Garguilo J, Nemanich RJ, Edwards GS, Simon JD. (2005) Oxidative potentials of human eumelanosomes and pheomelanosomes. Photochem Photobiol. 81:145-8.
Sandberg MA, Gaudio AR, Miller S, Weiner A. (1994) Iris pigmentation and extent of disease in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 35: 2734-40.
Sarna T. (1992) Properties and function of the ocular melanin: a photobiophysical view. J Photochem Photobiol. 12:215-58.
Sarna T, Swartz HA. (1998) The physical properties of melanin. In The pigmentary system: Physiology and pathophysiology. (Ed. Nordland JJ, et al.) Oxford Univ. Press, Oxford, p. 333-58.
Scotto J, Fraumeni JF, Lee JA. (1976) Melanomas of the eye and other noncutaneous sites: epidemiologic aspects. J Natl Cancer Inst. 56:489-91.
Shields JA, Shields SC. (1992) Intraocular tumor: A text and atlas. Saunders WB, Philadelphia, p. 54-306.
Singh AD, Topham A. (2003) Incidence of uveal melanoma in the United States: 1973-1997. Ophthalmology 110:956-61.
Swerdlow AJ. (1983) Epidemiology of eye cancer in England and Wales, 1962-1977. Am J Epidemiol. 118:294-300.
Takeuchi S, Zhang W, Wakamatsu K, Ito S, Hearing VJ, Kraemer KH, Brash DE. (2004) Melanin acts as a potent UVB photosensitizer to cause a novel mode of cell death in murine skin. Proc Natl Acad Sci USA. 101:15076-81.
Tuomaala S, Eskelin S, Tarkkanen A, Kivela T. (2002) Population-based assessment of clinical characteristics predicating outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 43:3399-408.
Tucker MA, Shields JA, Hartge P, Augsburger J, Hoover RN, Fraumeni JF. (1985) Sunlight exposure as risk factor for intraocular malignant melanoma. N Engl J Med. 313:789-92.
Vajdic CM, Kricker A, Giblin M, McKenzie J, Aitken J, Giles GG, Armstrong BK. (2001) Eye color and cutaneous nevi predict risk of ocular melanoma in Australia. Int J Cancer. 92: 906-12.
Wakamatsu K, Hu DN, McCormick SA, Ito S. (2008) Characterization of melanin in human iridal and choroidal melanocytes from eyes with various colored irides. Pigment Cell Res. 21:97-105.
Wang Z, Dillon J, Gaillard ER. (2006) Antioxidant properties of melanin in retinal pigment epithelial cells. Photochem Photobiol. 82:474-9.
Weis E, Shah CP, Lajous M, Shields JA, Shields CL. (2006) The association between host susceptibility factors and uveal melanoma: a meta-analysis. Arch Ophthalmol. 124:54-60.
Weiter JJ, Delori FC, Wing GL, Fitch KA. (1985) Relationship of senile macular degeneration to ocular pigmentation. Am J Ophthalmol. 99:185-7.
Wilkerson CL, Syed NA, Fisher MR, Robinson NL, Wallow IHL, Albert DM. (1996) Melanocytes and iris color: light microscopic findings. Arch Ophthalmol. 114:437-42.
Winkler BS, Boulton ME, Gottsch JD, Sternberg P. (1999) Oxidative damage and age-related macular degeneration. Mol Vis. 5:32.
Yanoff M, Duker JS. (1999) Ophthalmology. Mosby, London.
Yu GP, Hu DN, McCormick S, Finger PT. (2003) Conjunctival melanoma: Is it increasing in the United States? Am J Ophthalmol. 135: 800-6.
Yu GP, Hu DN, McCormick SA. (2006) Latitude and incidence of ocular melanoma. Photochem Photobiol. 82:1621-6.
2/18/09