BASIC PHOTOMOVEMENT
How Light Puts Living Microorganisms on the Right Track
Francesco Lenci
Istituto Biofisica CNR, Via G. Moruzzi 1 - 56100 Pisa (Italy)
francesco.lenci@pi.ibf.cnr.it
Introduction
Besides being a fundamental source of energy for all photosynthetic organisms and microorganisms, light is an environmental stimulus of primary importance for all living beings, terrestrial and aquatic, diurnal and nocturnal, prey and predator, alike. The importance of light as a stimulus applies for creatures provided with "eyes" and neural networks, as well as for aneural life forms like plants, fungi and even unicellular microorganisms, such as bacteria, algae and protozoa. In all living organisms, movement is one of the most important outcomes of the complex interaction and communication of an organism with the environment. The variety of movements resulting from the action of light is so diverse that it is difficult even to mention the most important of them.
The main body of this module is devoted to photomovements of freely motile microorganisms, but in the interest of completeness, several other fascinating light-controlled motile behaviors are presented in the next few paragraphs.
Fireflies: Male fireflies fly emit bioluminescent light flashes with a well-codified temporal structure that is specific to their species. Female fireflies, lying on the ground, perceive and respond to these signals setting up a light-based dialog, which allows the male to track and navigate to the female.
Birds: UV vision allows birds (as well as several invertebrates and some fish) to track the sun, helping them to migrate and relocate. Birds, in particular, perceive the plane of polarization of UV radiation, and use this to determine the proper direction for their migratory paths. In doing this they take advantage of the fact that shorter wavelengths of electromagnetic radiation (such as UV) penetrate atmospheric haze much better than longer wavelengths (such as visible light). These shorter wavelengths are also more polarized in the atmosphere than longer wavelengths, making this a good cue to guide migratory flights.
Plants and Fungi: In plants and fungi light provides information about the external environment, serving as a trigger for several sensory photobiological processes, including phototropism, photomorphogenesis, light-regulated intracellular redistribution of chloroplasts, photoinduced gene expression fern-spore germination and light-activated stomatal opening (Watanabe, 2003). Ambient light conditions are monitored by specialized photoreceptors, which include the red/far-red photoreversible phytochromes, the blue-light-absorbing cryptochromes and phototropins (Briggs and Huala, 1999; Lin, 2000; Furuya and Kuno, 2003; Bhoo and Song, 2003; Losi, 2007; Whitelam and Halliday, 2007; Franklin, 2008; Briggs, 2008, Kang et al., 2008; Losi and Gaertner, 2008; Nemhauser, 2008).
Phototropism, for example, is an oriented movement of plants and fungi, or their organs, toward (positive phototropism) or away from (negative phototropism) a light source (Galland, 2003; Kimura and Kagawa, 2007). The importance of these sensory reactions is reflected in the fact that one of them, for example, differential elongation in response to lateral differences in light quantity and/or quality, is ubiquitous in the plant kingdom, suggesting that this photosensory response provides an adaptive evolutionary advantage. Phototropins, one of the blue-light photoreceptors of higher plants, influences phototropism by monitoring the direction of light (Christie, 2007). Phototropism and photomorphogenesis are regulated by complex interactions among several photosensory systems. As a result of the interactions of these multiple photosensory systems, plants are able to maximize the adaptive advantage of the phototropic and photomorphogenetic response in ever changing light environments. Some aspects of these photosensory responses are also discussed in the Photomorphogenesis and Environmental Photobiology sections of Photobiological Sciences Online.
Light-induced chloroplast movements allow plant cells to perform highly efficient photosynthesis, both under low and high fluence rate conditions. Low Fluence Responses (LFR) cause chloroplasts to distribute at the cell surface closest to the light source so as to harvest as much light as possible, whereas High Fluence Responses (HFR) cause the chloroplasts to relocate themselves along the sides of the cell, so as to avoid photodamage (Wada and Kagawa, 2001; Watanabe, 2003, Wada et al., 2003; Suetsugu, and Wada, 2007).
Photobehavioral Responses of Microorganisims
Many freely motile microorganisms are provided with a photoreceptor apparatus able to perceive the quantity and the quality of light in the environment, and to transform the absorption of a photon into a biophysical or biochemical signal, which can be recognized, elaborated and transduced by the living system (Haupt, 1985; Song, 1983; Nultsch, 1991; Hegemann, 1997; Kawai and Kreimer, 2000; Haeder and Lebert, 2001; Lenci and Watanabe, 2001; Spudich et al., 2001; Sgarbossa, et al., 2002; Barsanti, et al., 2003; Checcucci, et al., 2003; Hendriks and Hellingwerf, 2003; Jung and Spudich, 2003; Marangoni, et al., 2003).
Thanks to this photosensory capability, spatial and temporal variations in the external light field can elicit modifications of movement patterns. In other words, light constitutes an information signal, which controls the movement and eventually guides the cells into the environmental niches in which the illumination conditions are the best for their growth, survival and/or development. It is important to notice that even microorganisms that, like ciliated protozoa, for instance, do not harvest and convert light energy directly for their metabolism, are able to perceive and react to photic stimuli to gather into habitats that can be unfavorable for their predators, and propitious for their prey, and in general, for food. Some colored ciliates, moreover, contain endogenous photosensitizer(s), generally used as defensive pigment(s) against predators, and even relatively dim light can cause severe damage, and their ability to escape lighted spots is directly linked to their survival. [See the Module on Photomovements of Ciliates.]
The main photobehavioral responses, according to the terminology of Diehn et al. (1977), can be divided into photophobic reactions and phototaxis. In photophobic responses, the sensory stimulus consists of a sudden change in light intensity, which elicits a transient variation in the motor activity of the microorganism. Step-up and step-down photophobic responses are caused by a step-wise increase or decrease, respectively, in photon flux. [Note that these terms do not refer to the change in motility. There can be an increase in motility, for example, in either a step-up or a step-down response.] In fact, usually both step-up and step-down photophobic responses produce a brief cessation of forward movement (stop response) followed by a random change of the direction of movement. The response pattern depends on the morphology of the microorganism, and is independent of light direction.
A photophobic response typically lasts for a few seconds, after which the microorganism can become adapted to the new illumination conditions. If the sudden change in light intensity is experienced because the cell crosses a light-dark border, the final outcome of the step-up photophobic response is an avoidance of the lighted region, and an accumulation in the shaded area (photodispersal). Usually step-up photophobic reactions occur with a time lag with respect to the stimulus application. This lag decreases with increasing photon flux density and depends on the stimulating wavelength. Similarly, in the case of a step-down photophobic response, the stimulus, now an abrupt decrease in light intensity, allows the cell to escape from shaded areas, and to accumulate in lighted regions (photoaccumulation). Figure 1 shows a schematic reconstruction of a step-up photophobic response.
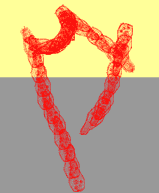
Figure 1. Schematic reconstruction of the step-up photophobic response of the ciliate Blepharisma japonicum crossing a dark-light border. Note that there are repeated changes of direction in the lighted, upper region. Once the organism crosses back into the shaded, lower region, this pattern ceases, and the organism continues to move on a straight path.
Phototaxis results from the detection of the direction of light propagation. It is a directional response. Phototaxis is defined as positive or negative according to whether the oriented movement is toward (+) or away from (-) the source. This directional response implies the existence of a very sophisticated photoreceptor apparatus. To perceive the position of the light source, the photoreceptor apparatus needs to be asymmetric. This asymmetry will allow the photoreceptor to sense the vectorial characteristics of the light signal. This asymmetry can be accomplished either by means of a single photosensing unit coupled to a screening device, which periodically shades the photoreceptor proper (a two-instant mechanism), or by the presence of two distinct differentially illuminated photoreceptor organelles (a one-instant mechanism). The essential feature of a two-instant mechanism is that light direction is perceived by comparing the light absorbed by one photoreceptive unit at two instants of time. If the light-screening device moves to a different position around the photoreceptor at these two instants of time, then the detected light signal will change dependent on its direction of propagation. A one-instant mechanism determines light direction by comparing the light absorbed at two photoreceptive regions of the cell at a single instant of time (Feinleib, 1985; see also the Module by K. W. Foster).
In photokinesis, the steady-state rate of movement depends on the absolute magnitude of the light intensity. If the steady-state velocity of a microorganism at a given light intensity is higher than in the dark control, photokinesis is called positive; if it is lower, photokinesis is called negative. When photokinesis is linked to the energy-producing photosynthetic apparatus (as is the case in many prokaryotes), this phenomenon cannot strictly be considered to be a "true" photosensory response.
It is worth noting that the same organism may be able to exhibit more than one photoresponse, which in some cases concur in causing the same result (e.g., photodispersal induced by negative phototaxis and step-up photophobic responses) and, in other cases, depend on the environmental illumination condition (e.g., positive or negative phototaxis elicited by low or high light intensities). Consequently, photoaccumulation and/or photodispersal of cell populations can be the result of phobic, kinetic and tactic responses, and drawing conclusions on behavioral strategies can often be rather difficult.
Monitoring Photomovement: An Experimental Challenge
By definition photomovement is motion that is influenced by light, but this presents an interesting experimental challenge. For an experimenter to see movement there must be sufficient light on the photomotile organism, but the light needed to see the organism can also change its pattern of photomovement. Figure 2 shows a schematic block diagram of a typical experimental apparatus for viewing photomotile responses in microorganisms without spuriously influencing the response. Infrared radiation, which is not perceived by the microorganisms under investigation, is used to illuminate the organisms being studied, and the images are recorded by an infrared video camera. This allows the operator to observe the cells without "disturbing" them.
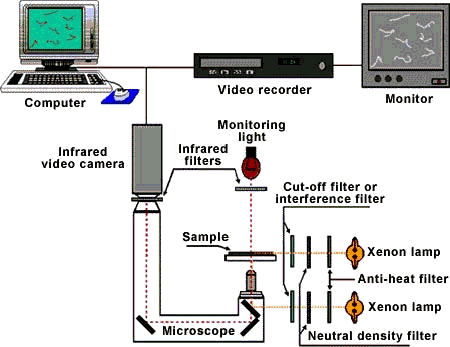
Figure 2. Block diagram of a typical experimental apparatus for measuring photomotile responses in microorganisms.
To quantitatively measure photoresponses, population methods as well as single cell track (usually computer-assisted) analysis are currently used. Both of them have advantages and drawbacks, and it should be emphasized that these measurements deserve an accurate choice of meaningful parameters to reliably discriminate among different reactions.
Photosensing Pigments and Phototransduction in Microorganisms
For some microorganisms both the structure of the photoreceptor pigment(s) as well as the primary molecular events that follow the absorption of light have been identified. But for many unicellular organisms even the chemical nature of the molecule acting as light detector is still doubtful. For these organisms, the mechanism by which the photoreceptors transmit information about the characteristics of a light signal to the downstream transduction pathway components is undetermined.
While many aspects of photosensory and phototransduction systems are still uncertain, considerable progress has been achieved in recent years. Multidisciplinary approaches that have yielded new information include selection and isolation of behavioral and pigment-lacking mutants (Spudich and Bogomolni, 1984; Chung et al., 2001; Bhaya et al., 2006), quantitative analysis of motile responses (Haeder and Vogel, 1991), steady-state and time-resolved spectroscopic and microspectroscopic studies of the candidate photosensing structures (Plaza et al., 2007; Sundstroem, 2008), and electrophysiological measurements (Woods, 2001; Sineshchekov and Spudich, 2005).
The main presently known photoreceptors that absorb in the blue region of the spectrum are cryptochromes, BLUF proteins, photoactivated cyclases, phototropins (all of them use flavins as chromophores), and the photoactive yellow proteins (using para-cumaric acid as the chromophore). Others absorb in the green and the yellow-orange (rhodopsins) and in the red (proteins whose prosthetic group are the hypericin-like chromophores stentorins, maristentorin or blepharismins). An updated and comprehensive review of the literature on photosensing pigments can be found in Watanabe (2003), Briggs and Spudich (2005), Spudich (2006), Losi (2007), Hegemann (2008), and for hypericin-like pigments, in Lobban et al. (2007).
It is worth noting that in one of the most extensively studied microorganisms, the photosynthetic flagellated alga Euglena, (see, e.g., Checcucci et al., 1976 and references therein), the available results are still conflicting, and the debate on the nature of its photoreceptor protein, flavoprotein vs. rhodopsin, is open. The old hypothesis of a flavin-type photoreceptor has recently acquired strong support by the discovery of a novel blue-light photoreceptor referred to as PAC, for Photoactivated Adenylil Cyclase (Isei et al., 2002; Ntefidou and Haeder, 2005; Ito et al., 2007), whereas quite recent results of Barsanti et al. (2008) confirm that the photocycle and the absorption spectra of the photoreceptor possess strong spectroscopic similarities with a rhodopsin-like protein (Barsanti et al., 2000).
The structural formulas of some of the most studied photosensing chromophores are reported in Figure 3. This wide variety of photosensing systems may have some interesting evolutionary implications. Apparently, the natural selection of photoreceptors did not stop at one of the very first efficient systems found, most probably the rhodopsin-like molecules of archaebacteria, despite the fact that this system demonstrates extreme sensitivity, low noise, extended dynamic range and light adaptation. Evolutionary forces have designed a large family of photoreceptors, each member of which suits the special requirements of a particular microorganism or group of microorganisms. In the archaebacteria, light sensing involves photoisomerization of a rhodopsin-like chromophore bound to a 7-helix transmembrane protein. In evolutionarily "newer" systems, other chromophores bound to other macromolecules sense light by a variety of mechanisms that may not involve photoisomerization. It seems that many systems with just adequate performance for perceiving and transducing light signals have been preserved during evolution.
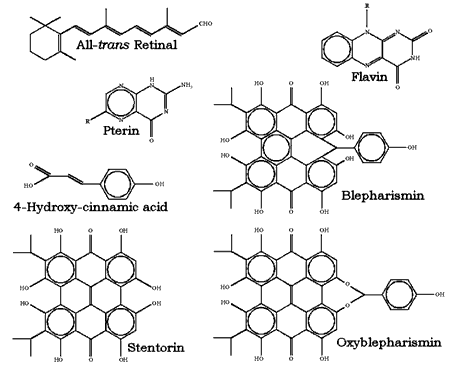
Figure 3. Chemical structure of the most studied chromophores of photoreceptors for photomotile responses of microorganisms.
There are many variations among the different photosensory systems. There are differences in the molecular structure of the photosensor. There are differences in their ability to perceive the various characteristics of photic stimuli (spectral composition, intensity, direction, polarization). There are also differences in the final alteration of the ciliary or flagellar beating pattern that is induced. But in all cases, the phototransduction mechanism is based on molecular events started by the modifications in the photoreceptive unit that are induced by light.
Several experimental techniques have been utilized to investigate the molecular and subcellular basis of the capacity of unicellular microorganisms (bacteria, microalgae, protozoa) to perceive environmental light stimuli and to respond to them by modifying their movement. In any light-induced motile reaction, the sequence of steps can be schematized as follows:
Photon absorption -->Because of their intrinsic multidisciplinary character, the problems of identification of the chromophore responsible for the photoresponse, and of the subsequent steps of the photosensory transduction chain in microorganisms, can be best faced using different experimental techniques and methodologies (Sgarbossa et al., 2002). A variety of approaches can allow the investigator to clarify single portions of this photosensory transduction chain: from spectroscopic and photophysical/photochemical techniques to chemical surgery, from electrophysiology to the use of specific mutants, just to mention a few, as schematically shown in Figure 4.
Photoreceptor light-induced modifications -->
Signalling state -->
Intracellular biological signal transduction -->
Motor apparatus pace alteration -->
Photomotile response
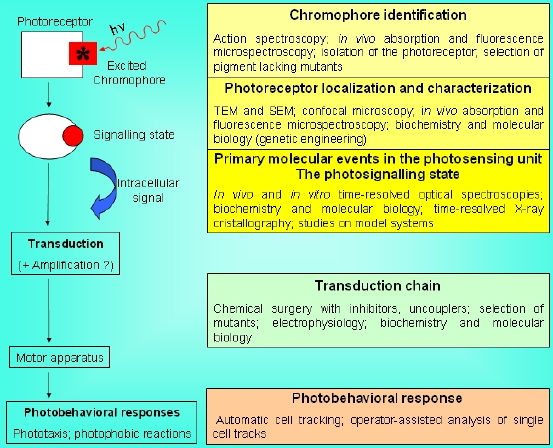
Figure 4. Simplified scheme of a photosensory transduction chain and of the most used experimental approaches to clarify the nature of the different functional/molecular steps and structural components.
In some cases the individual chromophores of photosensing systems can be loosely bound to flexible molecular frameworks, and isolated from each other. The spatial separation of the chromophores does not allow energy transfer among them, and the conformational mobility of the complex can give rise, upon absorption of even a single photon, to local rearrangements of the molecular domain around the chromophore. Alternatively, the light detecting pigments can be arranged in ordered rigid structures (see the case of Euglena below). In any case, the local perturbation occurring upon the absorption of a photon initiates a chain of molecular events, which are at the heart of the sensory transduction process, ultimately ending in a physiological response. Following the absorption of a photon, the photosensing chromophore undergoes modifications leading to the formation of the signaling state. This signaling state triggers a transduction chain eventually acting on the motor apparatus (Hellingwerf, 2000; Hendriks and Hellingwerf, 2003; van der Horst et al., 2007).
Photodetecting units can be assembled in ordered arrays, and in this case the molecular architecture functions to provide living cells with efficient photoreceptor-phototransducing systems. It can be seen in a variety of photosensing systems. Examples include the embedding of these systems in a membrane, as occurs in the thylakoids in chloroplasts or in the patches of sensory rhodopsins in Halobacteria. A similar close packing, but not in a membrane, can occur when photosensing systems are arranged in a quasi-crystalline arrangement in a subcellular organelle, as, e.g., in the ParaFlagellar Body of Euglena, shown in Figure 5.
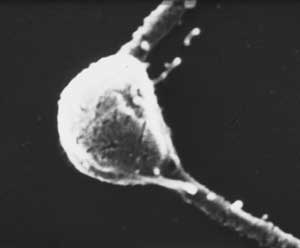
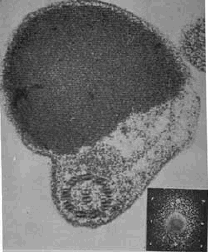
Figure 5. Euglena ParaFlagellar Body: (Left) Scanning Electron Micrograph (kindly provided by Paolo Gualtieri, CNR Institute of Biophysics, Pisa); (Right) Transmission Electron Micrograph (kindly provided by Ester Piccinni, Padova University).
Concluding Remarks
Hopefully this Module will create some curiosity so that people will read and enjoy the Advanced Modules. At present it seems as if every single unicell has its own system for photomovement, i.e., specific photosensing chromophores embedded in and interacting with particular molecular pockets, distinctive signaling states triggering unique and quite different molecular cascades. The question is then if the cause of such a multiplicity of interpretations is a consequence of our low level of knowledge and understanding or if it is an intrinsic feature of these natural phenomena.
In nature, of course, light is not the only environmental signal affecting the behavior of microorganisms, and their motile responses result from the integration of "internal needs" (such as, for instance, metabolism and cell cycle) with a diversity of external stimuli (chemical, mechanical, gravitational, photic,...). Integrated investigations of their motile responses to different environmental signals (chemical and photic, for example) might, therefore, offer clues for a deeper understanding of sensory processes in microorganisms.
Acknowledgments
I am deeply grateful to Giovanni Checcucci (Istituto Biofisica CNR, Pisa) for preparing the drawings for this module.
References
Barsanti, L., Coltelli, P., Evangelista, V., Passatelli, V., Frassanito, A. M., Vesentini, N., Santoro, F, Gualtieri, P. (2008) In Vivo Absorption Spectra of the Two Stable States of the Euglena Photoreceptor Photocycle, Photochem. Photobiol., in press
Barsanti, L., V. Evangelista, P. Gualtieri and V. Passarelli (2003) Photoreception in microalgae. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (121-1) - (121-14).
Barsanti, L., Passarelli, V., Walne, P. L. and Gualtieri, P. (2000) The photoreceptor protein of Euglena gracilis. FEBS Lett. 482, 247-251.
Bhaya D, Nakasugi K, Fazeli F, and Burriesci MS. (2006) Phototaxis and impaired motility in adenylyl cyclase and cyclase receptor protein mutants of Synechocystis sp. strain PCC 6803, J Bacteriol.,188, 7306-7310.
Bhoo, S. H. and P. S. Song (2003) Phytochrome: molecular properties. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (129-1) - (129-8).
Briggs, W.R. and E. Huala (1999) Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol. 15:33-62.
Briggs, W. R. and Spudich, J. L. (Eds., 2005) Handbook of Photosensory Receptors, Wiley
Briggs, W. R. (2008) In the Light of Day: Plant Photomorphogenesis, Mol Plant 2008 1: 2-3 and the entire issue of this new journal
Checcucci, A., Colombetti, G., Ferrara, R., and Lenci, F. (1976) Action spectra for photoaccumulation of green and colorless Euglena: evidence for identification of receptor pigments, Photochem. Photobiol. 23, 51-54
Checcucci, G., A. Sgarbossa and F. Lenci (2003) Photomovements in microorganisms: an introduction. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (120-1) - (120-10).
Christie, J. M. (2007) Phototropin Blue-Light Receptors, Ann. Rev. Plant Biol. 58, 21-45
Chung, Y., Cho, M., Moon, Y., et al.,(2001) Ctr1, a Gene Involved in a Signal Transduction Pathway of the Gliding Motility in the Cyanobacterium Synechocystis sp. PCC 680, FEBS Lett., 492, 33-38.
Diehn, B., Feinleib, M.E., Haupt, W., Hildebrand, E., Lenci, F. and Nultsch, W.: Terminology of behavioral responses of motile microorganisms, Photochem. Photobiol. 26 (1977) 559-560.
Feinleib. M. E. (1985) Behaviooral studies of free-swimming photoresponsive organisms. In Sensory perception and transduction in aneural organisms (G. Colombetti, F. Lenci and P. S. Song, Eds), Plenum Press, New York, pp. 119-146.
Franklin, K. A. (2008) Shade avoidance, New Phytologist, 179, 930-944.
Furuya, M. and N. Kuno (2003). Phytochrome geneaology. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, (130-1) - (130-8).
Galland, P. (2003) Phototropism. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (132-1) - (132-17).
Ghetti, F. and C. Bagnoli (2003) Environmental UV action spectroscopy. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (114-1) - (114-5).
Haeder, D. P. (2003) Photoecology and environmental photobiology. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (116-1) - (116-7).
Haeder, D. P., and M. Lebert (Eds.) (2001) Photomovements, Vol. 1 in Comprehemsive Series in Photosciences, (D.-P. Haeder and G. Jori, Ser. Eds.), Elsevier, Amsterdam.
Haeder, D. P., and Vogel, K. (1991) Simultaneous tracking of flagellates in real time by image analysis, J. Math. Biol. 30, 63-72.
Haupt., W. (1985) General survey of sensory transduction in aneural organisms. In Sensory perception and transduction in aneural organisms (G. Colombetti, F. Lenci and P. S. Song, Eds), Plenum Press, New York, pp. 1-18.
Hegemann, P. (1997) Vision in microalgae. Planta. 203, 265-274.
Hegemann, P. (2008) Algal Sensory Photoreceptors, Ann. Rev. Plant Biol. 59, 167-189.
Hellingwerf, K. J. (2000) Key issues in the photochemistry and signalling-state formation of photosensor proteins. J. Photochem Photobiol. B:Biol. 54, 94-102.
Hendriks, J. and K. J. Hellingwerf, (2003) Photoactive Yellow Protein, the prototype xanthopsin. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (123-1) - (123-22).
van der Horst, M. A., Arents, J. C., Kort, R., and Hellingwerf, K. J. (2007) Binding, tuning and mechanical function of the 4-hydroxy-cinnamic acid chromophore in photoactive yellow protein, Photochem. Photobiol. Sci, 6, 571-579.
Iseki, M., S. Matsunaga, A. Murakami, K. Ohno, K. Shiga, K. Yoshida, M. Sugai, T. Takahashi, T. Hori and M. Watanabe (2002) A blue-light-activated adenylil cyclase mediates photoavoidance in Euglena gracilis. Nature 415, 1047-1051.
Ito, S., Murakami, A., Sato, K., Nishina, Y., Shiga, K.,Takahashi, T., Higashi, S., Iseki, M. and Watanabe, M. (2005) Photocycle features of heterologously expressed and assembled eukaryotic flavin-binding BLUF domains of photoactivated adenylyl cyclase (PAC), a bluelight receptor in Euglena gracilis. Photochem. Photobiol. Sci. 4, 762-769.
Jung, K.-H. and J. L. Spudich (2003) Microbial rhodopsins: transport and sensory proteins throughout the three domains of life. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (124-1) - (124-11).
Kawai, H. and G. Kreimer (2000) Sensory mechanisms--phototaxes and light perception in algae. In:The Flagellates (B. S. C. Leadbeater and J. C. Green, Eds.). pp. 124-146. Taylor and Francis, London.
Kang, B. Grancher, N., KoyVmann, V., Lardemer, D., Burney, S., and Ahmad, M. (2008) Multiple interactions between cryptochrome and phototropin blue-light signalling pathways in Arabidopsis thaliana Planta 227, 1091-1099.
Kimura, M. and Kagawa, T. (2007) Phototropin and light-signaling in phototropism. Current Opinion in Plant Biology, 9, 503-508.
Lenci, F. and M. Watanabe (2001) Photomovements and light-sensing mechanisms. In Photobiology for the 21st Century, (D. Valenzeno and T. Coohill, Eds.) pp. 211-219.
Lin, C. (2000) Plant blue-light receptors. Trends Plant Sci., 5, 337-342.
Lobban, C. S., Hallam, S. J., Mujherjee, P., and Petrich, J. W. (2007) Photophysics and Multifunctionality of Hypericin-Like Pigments in Heterotrich Ciliates: A Phylogenetic Perspective. Photochem. Photobiol., 83, 1074-1094.
Losi, A. (2007) Flavin-based Blue-light Photosensors: A Photobiophysics Update, Photochem. Photobiol. 83, 1283-1300.
Losi, A., and Gaertner, W. (2008) Shedding (blue) light on algal gene expression, Proc. Natl. Acad. Sci. 105, 7-8.
Marangoni, R., S. Lucia and G. Colombetti (2003) Photomovements in ciliates. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (122-1) - (122-19).
Nemhauser, J. L. (2008) Dawning of a new era: photomorphogenesis as an integrated molecular network Current Opinion in Plant Biol. 11, 4-8.
Ntefidou, M. and Hader, D. P. (2005) Photoactivated adenylyl cyclase (PAC) genes in the flagellate Euglena gracilis mutant strains, Photochem. Photobiol. Sci. 4, 732-739.
Nultsch, W. (1991) Survey of photomotile responses in microorganisms. In Biophysics of photoreceptors and photomovements in microorganisms. (F. Lenci, F. Ghetti, G. Colombetti, D.-P. Haeder and P.-S. Song, Eds), Plenum Press, New York, pp. 1-5.
P. Plaza, P, Mahet, M., Martin, M. M., Checcucci, G. and Lenci, F. (2007) Target analysis of primary processes involved in the Oxyblepharismin-Binding Protein, J. Phys. Chem. B, 111, 690-696. Sgarbossa, A., G. Checcucci, and F. Lenci (2002) Photoreception and photomovements of microorganisms, Photochem. Photobiol. Sci., 1, 459-467.
Sineshchekov, O. A., and Spudich, J. L. (2005) Sensory Rhodopsin Signaling in Green Flagellate Algae, in "Handbook of Photosensory Receptors" (Briggs, W. R., and Spudich, J. L., Eds), pp. 25-42.
Song, P.-S.: Protozoan and related photoreceptors: Molecular aspects. Annu. Rev. Biophys. Bioeng. 12 (1983) 35-68.
Spudich, J. L. (2006) The multitalented microbial sensory rhodopsins, Trends Microbiol. 14, 480-487.
Spudich JL, and Bogomolni RA. (1984) Mechanism of colour discrimination by a bacterial sensory rhodopsin. Nature. 312, 509-513.
Spudich J.L., C. S. Yang, K. H. Jung KH and E. N. Spudich (2001) Retinylidene proteins: structures and functions from archaea to humans.Annu Rev Cell Dev Biol. 16, 365-92 Suetsugu, N, and Wada, M. (2007) Chloroplast photorelocation movement mediated by phototropin family proteins in green plants Biological Chemistry, 388, 927-935.
Sundstroem, V. (2008) Femtobiology, Ann. Rev. Phus. Chem. 59, 53-77.
Wada, M. and T. Kagawa (2001) Light controlled chloroplast movements. In Photomovements, Vol. 1 in Comprehensive Series in Photosciences, (D.-P. Haeder and G. Jori, Ser. Eds.), Elsevier, Amsterdam. pp. 897-924.
Wada, M., Kagawa. T, and Sato, Y. (2003) Chloroplast Movement. Annu. Rev. Plant Biol. 54, 455-68.
Watanabe, M. (2003) Action spectroscopy for photosensory processes. In Handbook of Organic Photochemistry and Photobiology, 2nd Edition (W. M. Horspool and F. Lenci, Eds), CRC, Boca Raton, pp. (115-1) - (115-16).
Whitelam, G. C. and Halliday, K. J.,Eds. (2007) "Light and Plant Development" - Ann. Plant Rev., Blackwell Publ.
Wood D. C. (2001) Electrophysiology and light responses in Stentor and Blepharisma. In Photomovements (D.-P Hader and M. Lebert, Eds.), Comprehensive Series in Photosciences (D.-P. Hader and G. Jori, Eds.), Vol 1, pp. 505-518.
9/17/08