BASIC PHOTOSENSITIZATION
Nancy L. Oleinick
Department of Radiation Oncology
Case Western Reserve University School of Medicine
Cleveland, Ohio 44106-4942
nlo@case.edu
What is Photosensitization?
Photosensitization is a reaction to light that is mediated by a light-absorbing molecule, which is not the ultimate target. Photosensitization can involve reactions within living cells or tissues, or they can occur in pure chemical systems. In photobiology, we are concerned with the reactions in living systems.
Recall that the First Law of Photochemistry states that light (photons) must be absorbed to have an effect. In some cases, the molecule that absorbs a photon is altered chemically, but does not change any other molecule in the system. In other cases, the molecule that absorbs the photon ultimately alters another molecule in the system. In the latter process (photosensitization), the molecule that absorbs the photon is called the photosensitizer (or simply sensitizer), and the altered molecule is the acceptor or substrate. Both the photosensitizer and light are required for photosensitization.
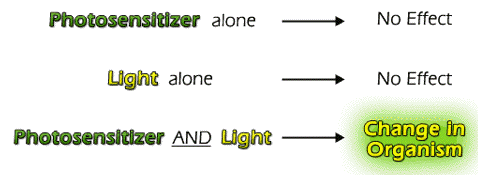
Photosensitizers are not usually consumed during photosensitization reactions. They return to their original state once the photosensitization reaction is complete. Photosensitizers can be photobleached, a chemical reaction following the absorption of light that produces a different molecular form, which does not absorb light, but this process typically competes with the photosensitization reaction.
Photosensitizers can be endogenous in living systems, or they can be taken up from exogenous sources. Endogenous photosensitizers include molecules such as porphyrins, bilirubin or chlorophyll, although in their normal cellular environment their potential photosensitizing effects are not apparent, either because their concentrations are too low, or because the molecules are sequestered in complexes that inhibit photosensitization reactions. Exogenous photosensitizers are numerous and include many varieties of dyes and biomolecules; some are natural products, e.g., from plants, and many others have been produced synthetically for medical, agricultural, or other uses. A few examples will be discussed below.
An ingenious variant on the endogenous/exogenous theme is the administration of an inert precursor, which is converted by the organism's metabolism into a fully functional photosensitizer. For example, many plants and animals will metabolize the precursor,
 -aminolevulinic acid, to the photosensitizer protoporphyrin. Protoporphyrin is a normal intermediate in biosynthetic pathways to produce cytochromes, hemoglobin (the oxygen-binding protein in red blood cells), and myoglobin, which binds oxygen in muscle. Under normal conditions the concentrations of free protoporphyrin are too low to produce photosensitization reactions, but if excess
-aminolevulinic acid, to the photosensitizer protoporphyrin. Protoporphyrin is a normal intermediate in biosynthetic pathways to produce cytochromes, hemoglobin (the oxygen-binding protein in red blood cells), and myoglobin, which binds oxygen in muscle. Under normal conditions the concentrations of free protoporphyrin are too low to produce photosensitization reactions, but if excess 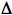 -aminolevulinic acid is provided, or if there is an impairment of normal metabolic pathways, protoporphyrin may accumulate, and photosensitivity reactions can be produced. The latter metabolic impairment is the root cause of the class of diseases known as the porphyrias.
-aminolevulinic acid is provided, or if there is an impairment of normal metabolic pathways, protoporphyrin may accumulate, and photosensitivity reactions can be produced. The latter metabolic impairment is the root cause of the class of diseases known as the porphyrias.
How Does Photosensitization Proceed?
In photosensitized reactions, the absorbed photon excites the sensitizer (abbreviated Sen in Figure 1) to one or more energy-rich state(s) (indicated by an asterisk, Sen*). The excited Sen* undergoes internal reactions that ultimately result in the chemical alteration of the substrate. This can occur in one of two types of reactions (Figure 1).
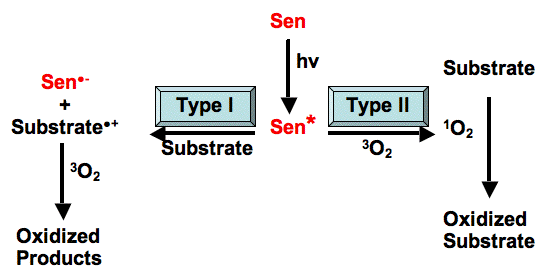
Figure 1. Type I vs. Type II Photosensitization Reactions.
In the Type I reaction, the excited sensitizer reacts directly with the substrate, in a one-electron transfer reaction, to produce a radical or radical ion in both the sensitizer and the substrate. Although electron transfer can proceed in either direction, usually the substrate donates an electron to the sensitizer, resulting in a substrate radical cation (Substrate.+), and a sensitizer radical anion (Sen.-). In the presence of oxygen, both of these radicals can further react to produce oxygenated products. This type of reaction can lead to a loss of the sensitizer, since it is converted to an oxidized molecule. Another possible reaction is the direct transfer of the extra electron of Sen.- to oxygen to produce the superoxide radical anion (O2.-), and regenerating the original sensitizer (Sen).
In the Type II reaction, the excited sensitizer transfers its excess energy to ground-state molecular oxygen (3O2), producing excited state singlet oxygen (1O2), and regenerating the ground-state sensitizer. Singlet oxygen then reacts with the substrate to generate oxidized products. The photosensitizer is not consumed during this type of photosensitized reaction.
How do Photosensitizers Produce Chemical Changes in Biological Tissues?
The transfer of an electron between a photosensitizer and the substrate (the Type I reaction) results in the creation of products that have an uneven number of electrons. Such radical species are often highly reactive. Radicals can further react with additional biological substrates producing changes in structure and/or function. Superoxide and hydroxyl radicals are important radical species that are often produced by Type I reactions in biological environments. For example, Malachite Green is marketed as a photosensitizer that produces biological effects via hydroxyl radicals. Because Type I reactions require a direct interaction of the photosensitizer and the substrate, they are favored by high substrate concentrations. They are also favored by low oxygen concentrations, since oxygen competes with the substrate for interaction with the photosensitizer.
In the Type II reaction, the transfer of energy from the photosensitizer to oxygen produces an excited singlet state of oxygen, appropriately called singlet oxygen. Typically, photosensitizers, like most other molecules, are in a singlet state in their normal ground state (see Figure 2). When they absorb light they are converted to a different singlet state with a greater energy content. Because the excited singlet state has a very short life-time, there is little opportunity for it to react with another molecule via either electron or energy transfer. For most photosensitizers, the excited singlet state rapidly undergoes a process known as intersystem crossing, in which there is a transition to a slightly lower energy level as the spin of an electron flips. The spin flip puts the molecule into a triplet energy state. Because transitions between triplet and singlet energy states are relatively forbidden, the excited triplet state tends to have a longer lifetime than an excited singlet state, and hence is more likely to enter into an energy transfer reaction. Singlet oxygen is a highly reactive oxygen species that has an excited state lifetime of a few microseconds in most biological environments, e.g., 3 to 4.5 µs in water. The Type II (singlet oxygen) mechanism is favored over the Type I (radical) mechanism by lower substrate concentration and higher oxygen concentration.
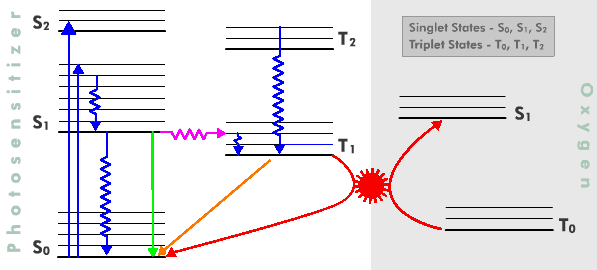
Figure 2. Jablonski Diagram depicting electronic transitions following the absorption of light by a photosensitizer, and energy transfer to an oxygen molecule, producing singlet oxygen. There is an implied vertical energy scale in this diagram such that higher electronic energy levels are above lower energy levels. Also, triplet states are drawn to the right of singlet states, and states involving oxygen are to the right of those involving the photosensitizer. The basic electronic transitions illustrated on the diagram are:
Absorption (blue solid arrows): Transfer of energy from a photon of light to a molecule, exciting the molecule. For absorption to occur, the energy of the photon must correspond to the energy difference between the ground state (S0) of the absorbing molecule and one of its excited states (S1 or S2).
Internal conversion (blue jagged arrows): Transitions of a molecule between electronic states having similar electronic spin, i.e., between singlet states or between triplet states.
Fluorescence (solid green arrow): Emission of energy of excitation from a molecule in the form of light. The energy of the emitted photon corresponds to the energy difference between the emitting and final states of the fluorescing molecule. The emitting and final states must have similar electronic spin states; i.e., they must both be singlet (or triplet) states.
Intersystem Crossing (purple jagged arrow): Transition of a molecule between electronic states having different electronic spin; i.e., from a singlet state to a triplet state, or vice versa.
Phosphorescence (gold solid arrow): Like fluorescence, this is emission of energy of excitation from a molecule in the form of light. The energy of the emitted photon corresponds to the energy difference between the emitting and final states of the phosphorescing molecule. Unlike fluorescence, the emitting and final states must have different electronic spin states, i.e., one is typically a singlet state and the other a triplet state.
Energy Transfer (red arrows): Electronic energy of one molecule, in this case the photosensitizer, is transferred to another molecule, in this case molecular oxygen. During the transfer, denoted in the figure by the “gear” connecting the two curved arrows, the triplet excited state of the photosensitizer is de-excited to the ground singlet state. At the same time, ground state molecular oxygen (one of the few molecules whose ground state is a triplet) is elevated to the first excited singlet state; so-called singlet oxygen. Note that both transitions involve a spin change of an electron.
(See the Jablonski Diagram (Figure 4) in Basic Photophysics for a simpler version of this figure, without oxygen.)
Many important photosensitization reactions are Type II processes, and the study of the biological effects of singlet oxygen produced via photosensitization reactions is an important area of research in modern photobiology. Ground-state molecular oxygen is relatively unreactive and diffuses rapidly through most biological environments, including cell membranes, which normally act as barriers for many substances. In contrast, singlet oxygen reacts with certain biological substrates, such as lipids and proteins, restricting the ability of singlet oxygen to diffuse great distances (Figure 3). Thus, Type II photosensitization can affect biological substrates at only a moderate distance from the photosensitizer itself, a distance that has been estimated to be anywhere from 0.02 micrometers to 0.15 micrometers. This can be contrasted with the hydroxyl radical, which is so reactive that it typically interacts with the first molecule it encounters, restricting its effects to the location where it is generated.
In either case, the limited distance over which intermediates like hydroxyl radical and singlet oxygen can produce effects means that the localization of photosensitizer molecules becomes important to their eventual biologic effects. For example, a photosensitizer that is very lipid soluble will tend to accumulate in the lipid interior of cell membranes, an environment in which singlet oxygen has a lifetime about twice as long as it does in an aqueous environment. When illuminated, the photosensitizer is likely to cause reactions with molecules in the membrane, rather than molecules in the cytoplasm. A similar localization phenomenon for some kinds of photosensitizers is the basis for the photodynamic therapy of cancer, a strategy that uses photosensitizers that tend to be retained in malignant tumors more avidly than in normal tissues. In some cases, photosensitizers can even be targeted to affect specific sites by attaching them to other molecules that localize at specific places in cells or tissues. Targeting and differential photosensitivity are requirements for most modern applications of photosensitization, as discussed below.
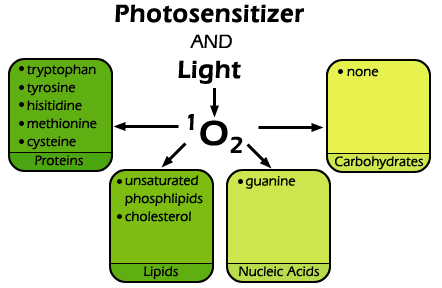
Figure 3. Singlet oxygen reacts readily with many biological substrates including certain amino acids in proteins, mainly tryptophan, tyrosine, histidine, cysteine and methionine. It also reacts with the guanine bases of DNA and RNA, as well as a variety of unsaturated lipids, including cholesterol and unsaturated fatty acids. It does not, however, affect carbohydrates appreciably.
The term photodynamic action was coined near the turn of the 20th century to refer to a light-driven reaction in which a photosensitizer molecule reacts in an oxygen-dependent manner with a substrate, to produce a change in the substrate. While it is often used to refer to Type II reactions, its original definition can also include Type I reactions that involve oxygen. Note that oxygen is an important reactant in most photosensitization reactions, whether it is part of the initial reaction with the excited photosensitizer (Type II), or reacts with later products to "fix" the initial radicals as oxidized products (Type I).
Why Aren't All Light-Absorbing Molecules Photosensitizers?
Most molecules that absorb light, and hence acquire energy in the process, subsequently lose the acquired energy through radiationless decay (Figure 2). That is, they give off heat in the process known as internal conversion. Photosensitizers, on the other hand, are molecules in which internal conversion is not efficient. Rather they transfer an electron to or from another molecule, or they transfer their energy of excitation to other molecules, often to molecular oxygen. In most cases, the reason that some molecules are so effective at electron transfer and/or energy transfer is that they very efficiently populate their excited triplet states. The relatively longer lifetime of the triplet state allows more time for energy and/or electron transfer to occur. So, most highly effective photosensitizers are characterized by high quantum yields for the production of their excited triplet state.
How Can Type I Photosensitization Reactions be Distinguished From Type II Reactions?
Distinguishing Type I from Type II reaction mechanisms is not always an easy task, particularly in biological systems. The simplest methods involve the use of additives that either prolong or shorten the excited state lifetime of singlet oxygen. Azide ion is a commonly used quencher of singlet oxygen. It interacts with singlet oxygen, rapidly converting it to ground state molecular oxygen before singlet oxygen can react with another molecule in the system. It is predominantly a physical quencher, a molecule that does not chemically react in the quenching process, unlike chemical quenchers. Thus, azide inhibition of a photosensitization reaction can provide evidence in support of a singlet oxygen mechanism. However, adequate controls are important, especially for azide. Azide might also interact with the photosensitizer itself, quenching the triplet state of the photosensitizer. This would inhibit any photosensitization resulting from the photosensitizer, not just a singlet oxygen process. An interaction of azide to quench the photosensitizer triplet state can be detected by measuring phosphorescence from the photosensitizer. Since phosphorescence results from the decay of the triplet state, any change in the number of photosensitizer molecules in the triplet state will change the phosphorescence. Thus, if azide does not alter the phosphorescence spectrum of the photosensitizer, but inhibits the observed biological photosensitization, then a singlet oxygen mechanism is suggested. When azide is added to cells, it can have other, non-photochemical effects, such as the inhibition of mitochondrial electron transport and superoxide dismutase; such effects can alter the end biological result, complicating the interpretation of the experiment.
An example of a chemical quencher of singlet oxygen is the amino acid histidine. Singlet oxygen can react chemically with the imidazole ring of histidine to produce oxidized products. Therefore, inhibition of a photosensitization reaction with histidine can suggest the intermediacy of singlet oxygen. However, histidine can also react with other oxygen radicals, so inhibition by histidine is not absolute proof that singlet oxygen was the active agent.
Further confirmation of the intermediacy of singlet oxygen can be obtained by replacing water with deuterium oxide. The lifetime of singlet oxygen is increased as much as 15-fold in deuterium oxide, compared to water. However, this 15-fold enhancement can be achieved only if there are no substrates for reaction with singlet oxygen. In biological environments, this is never the case, and it is almost never possible to completely replace water by deuterium oxide. Thus, it is customary to simply look for an enhancement of the observed biological reaction in deuterium oxide. Often this is coupled with an observation of an inhibition of the reaction by a quencher, such as azide, as independent evidence in favor of a singlet oxygen mechanism.
The generation of singlet oxygen by a photosensitization reaction can be confirmed by detecting light emission at 1270 nm, produced by the spontaneous decay of singlet oxygen to its ground state. This infrared emission can even be used to quantitate the amount of singlet oxygen being produced, but the method is limited by the very short lifetime of singlet oxygen in most biological environments. Newer instrumentation has been developed with very fast reaction times that can measure the 1270-nm luminescence, and allow the detection of singlet oxygen in biological systems. Such measurements can confirm the presence of singlet oxygen in a system, but can't give mechanistic information, since the singlet oxygen molecules detected by their luminescence represent the fraction that does not react with biological substrates.
Analysis of the products of singlet oxygen attack can also provide evidence for a Type II mechanism. In particular, when singlet oxygen reacts with cholesterol, it produces a product that is different from the products produced when any other species of oxygen reacts with cholesterol. This 5-
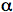 -hydroperoxide of cholesterol can be isolated from cell membranes as an indicator of the action of singlet oxygen.
-hydroperoxide of cholesterol can be isolated from cell membranes as an indicator of the action of singlet oxygen.
The direct detection of the Type I (radical) mechanism is typically more technically demanding, requiring electron spin resonance measurements to detect radical species.
Why is Photosensitization Important?
There are many ways in which photosensitization reactions impact our lives. For example, they are used in synthetic chemistry to produce products that would be much more difficult or expensive to produce by other means. However, in the following we will provide a few key examples of the biological relevance of photosensitization reactions. You will encounter many more examples, and more complete coverage of the examples below in other modules of this compendium. As previously discussed, a key feature of most biological applications of photosensitizers is the differential photosensitivity of targets. In addition the requirement for both photosensitizer and light to produce an effect allows photosensitization reactions to be better controlled than conventional chemical reactions.
Photosensitization of viruses in blood banks. There is great concern for the safety of blood that is used for transfusions. The potential for transmission of diseases, especially by viral contamination, is an ongoing concern. Photosensitizers have been studied for decontaminating blood in blood banks. This method capitalizes on the ability of certain photosensitizers to kill viruses (and other pathogens) at treatment levels that cause little damage to blood cells or plasma. In spite of the demonstrated efficacy, photo-decontamination processes have not yet become routine in blood banks.
Photosensitization of Cells in Photodynamic Therapy. Photosensitizers and light are also being used to kill malignant tumors, and to destroy other unwanted tissue in a process referred to as photodynamic therapy, or PDT. Again, differential photosensitivity is important, this time relying on the ability to cause greater photosensitization of malignant tumors than of surrounding normal tissues. For example, some photosensitizers used for PDT are retained by malignant tissues much longer than they are retained in normal tissues. Thus, one waits a predetermined time after injection of the photosensitizer, when normal tissues no longer retain much photosensitizer, and are not very photosensitive. Additional selectivity is obtained by illuminating just the malignant tumor, resulting in its selective destruction. In some cases,
 -aminolevulinic acid, a precursor of the natural photosensitizer, protoporphyrin, is administered topically to a skin lesion, and time is allowed for the precursor to be converted by the cells' own metabolism into the photosensitizer. Then the lesion is photoirradiated. One advantage of this type of photosensitization is that tumors, and some skin lesions, produce the photosensitizer more efficiently than the nearby normal cells, providing good selectivity of the response. An example of treatment of a skin tumor by PDT is shown in Figure 4.
-aminolevulinic acid, a precursor of the natural photosensitizer, protoporphyrin, is administered topically to a skin lesion, and time is allowed for the precursor to be converted by the cells' own metabolism into the photosensitizer. Then the lesion is photoirradiated. One advantage of this type of photosensitization is that tumors, and some skin lesions, produce the photosensitizer more efficiently than the nearby normal cells, providing good selectivity of the response. An example of treatment of a skin tumor by PDT is shown in Figure 4.
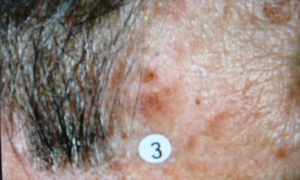
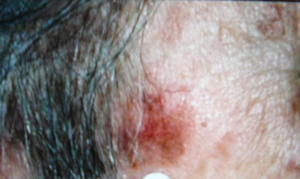
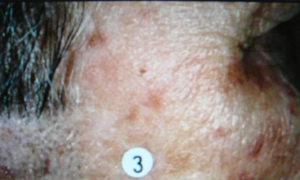
Figure 4. Photodynamic Therapy for Skin Cancer. The top image shows a malignant skin lesion just above the circled number 3. A photosensitizer was administered to this patient, and at an appropriate time afterward the lesion was illuminated with a laser emitting red light at 630 nm, a wavelength that is absorbed by the photosensitizer. The middle picture shows the lesion immediately after this illumination period. You can see the reddening that is induced by the treatment, as malignant cells are being lethally injured. The reddening gradually clears, leaving behind only normal skin cells, as shown in the bottom picture.
Another example of the use of PDT is for the elimination of abnormally growing blood vessels, which cause blindness in the disease macular degeneration. In this case, the photosensitizer, known as Verteporfin, is injected, and after a very short time, when the photosensitizer has not yet left the circulation, laser irradiation at 690 nm, a wavelength efficiently absorbed by Verteporfin, is directed precisely onto the abnormal blood vessels of the eye. The photosensitization of the undesired blood vessels destroys them, and prevents further loss of vision. Currently, several newer photosensitizers are available that absorb light very efficiently in the red region of the spectrum, a spectral region that permits greater tissue penetration of light. Research is aimed at defining the best way to use these photosensitizers for treatment of cancer and non-cancerous conditions. For each photosensitizer and disease, it is necessary to define the parameters of light intensity, duration of illumination, dose and timing of the photosensitizer, etc., which produce the best tumor response.
Photosensitization of plants and animals by photodynamic pesticides. Photosensitizers are helping to resolve problems that are of concern to both agriculturalists and urban dwellers, i.e., safe insecticides. A good example of this is the use of a photosensitizer and light to combat the Mediterranean Fruit Fly. Considerable public unrest has occurred in reaction to the spraying of Malathion over populated areas in California to contain the spread of this serious agricultural pest. An alternative that has worked very well in field tests is to use a photosensitizer combination mixed with a bait that attracts the fruit flies to small bait stations, typically hung from trees. Not only is the insecticide restricted to the bait stations, but the major constituent in these formulations is usually a dye that has been approved for use in food by the Food and Drug Administration of the United States. Nonetheless, when fruit flies consume the photosensitizer they die as they fly into the sunset.
Photosensitization of plants and animals by naturally occurring photosensitizers. Plants synthesize many photoactive compounds that can be toxic to insects, nematodes (roundworms), many animals, and even to other plants. Hypericin is one of the most toxic of this class of photosensitizers. It is produced by St. John's Wort (Figure 5), and is responsible for the photosensitivity sometimes seen as a side-effect of taking preparations made from this plant. It is also responsible for the sometimes severe phototoxicity that occurs in grazing livestock that wander into patches of this weed. Sheep and cattle are well-known to be affected. Interestingly, hypericin is being studied as a photosensitizer for PDT, to determine its effectiveness as an anti-tumor agent.
 -Terthienyl, another plant-derived photosensitizer, is responsible for the potent ability of some plant species to kill nematodes. However, both hypericin and
-Terthienyl, another plant-derived photosensitizer, is responsible for the potent ability of some plant species to kill nematodes. However, both hypericin and 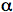 -terthienyl are toxic to many kinds of plants and animals, thus limiting their commercial usefulness. On the other hand, there does appear to be a commercial use for other plant-derived photosensitizers, including pigments produced for photosynthesis.
-terthienyl are toxic to many kinds of plants and animals, thus limiting their commercial usefulness. On the other hand, there does appear to be a commercial use for other plant-derived photosensitizers, including pigments produced for photosynthesis.

Figure 5. St. John's Wort. This plant produces a beautiful yellow flower as well as a powerful photosensitizer, hypericin. Cattle that graze on St. John's Wort become photosensitive, with sunburn like lesions on their hides.
Chlorophyll is a porphyrin-like molecule, and as such, can act as a photosensitizer. Plants must defend themselves against photosensitization by chlorophyll and other porphyrins that are precursors in the synthetic pathway leading to chlorophyll. One mechanism that they use to accomplish this protection is to minimize the concentrations of photosensitizing precursors by rapidly converting them to the next product in the chain of reactions leading to chlorophyll. If this rapid conversion is inhibited, or if its capability is overwhelmed, toxic levels of photosensitizing porphyrin intermediates can accumulate. This is the idea behind the use of so-called "laser" herbicides. Plants are treated with
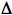 -aminolevulinic acid, the natural porphyrin precursor mentioned above, and 2,2'-dipyridyl, a synthetic iron chelator that inhibits some iron-containing enzymes. Protoporphyrin is one of the major species that accumulates in many plant species treated in this way. Interestingly, the application of protoporphyrin itself to plants does not result in photosensitivity, apparently because the protoporphyrin is not taken up into the proper cellular compartment in sufficient concentration to cause damage. Similar mechanisms of porphyrin accumulation as a result of ingesting
-aminolevulinic acid, the natural porphyrin precursor mentioned above, and 2,2'-dipyridyl, a synthetic iron chelator that inhibits some iron-containing enzymes. Protoporphyrin is one of the major species that accumulates in many plant species treated in this way. Interestingly, the application of protoporphyrin itself to plants does not result in photosensitivity, apparently because the protoporphyrin is not taken up into the proper cellular compartment in sufficient concentration to cause damage. Similar mechanisms of porphyrin accumulation as a result of ingesting 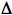 -aminolevulinic acid and 2,2'-dipyridyl can cause phototoxicity in insect larvae. This is the same
-aminolevulinic acid and 2,2'-dipyridyl can cause phototoxicity in insect larvae. This is the same 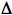 -aminolevulinic acid that is used for PDT in humans.
-aminolevulinic acid that is used for PDT in humans.
Conclusions
Photosensitization reactions form the basis for many of the photobiological effects that are presented in subsequent modules. They are of considerable importance in modern society, as indicated by the examples cited above, and are likely to become even more important as new uses and applications of these very controllable reactions are developed.
Suggested Readings
Anderson, F. Alan , Andrija Kornhauser, Constantine Zervos, Eds, Photochemical Toxicity: Toxic, Allergic, and Carcinogenic Aspects with Emphasis on Predicting Effects in Humans. In: J. Nat'l Cancer Inst., 69(1), July 1982.
Blum, Harold Francis, Photodynamic Action and Diseases Caused by Light. Reinhold Publ. Corp., New York, 1941.
Dean, Geoffrey. The Porphyrias: A Story of Inheritance and Environment. J. B. Lippincott Co., Philadelphia, Montreal, 1971.
Heitz, J.R. and K.R. Downum, eds, Light-Activated Pest Control. Amer. Chem. Soc. Books, Washington, DC, 1995.
Redmond, Robert W., Photophysics and Photochemistry in Photodynamic Therapy, In: Advances in Photodynamic Therapy: Basic, Translational, and Clinical, Michael R. Hamblin and Pawel Mroz, eds., Artech House, Boston and London, 2008, pp., 41-58.
Spikes, John D., Photosensitization. In: The Science of Photobiology, Kendric C. Smith, ed. Plenum Press, New York and London, 1989, pp. 79-110.
Valenzeno, Dennis Paul, Membrane Photomodification. In: Photobiological Techniques, D.P. Valenzeno, R.H. Pottier, P. Mathis and R.H. Douglas, eds. Plenum Press, New York and London, 1991, pp. 99-115.
04/06/10
09/09/11