DNA-PROTEIN CROSSLINKS
Kendric C. Smith1 and Martin D. Shetlar2
1Emeritus Professor, Radiation Oncology (Radiation Biology)
Stanford University School of Medicine
800 Blossom Hill Road, Unit R169, Los Gatos, CA 95032
kendric@stanford.edu
web.stanford.edu/~kendric/
2Professor Emeritus of Chemistry and Pharmaceutical Chemistry
School of Pharmacy, Room S-822
University of California, Box 0912
513 Parnassus Avenue
San Francisco, CA 94143-0912
shetlar@cgl.ucsf.edu
Introduction
Deoxyribonucleic Acid (DNA) in living cells is associated with a large variety of proteins. Therefore, it is logical to assume that the ultraviolet (UV) irradiation of cells could lead to reactive interactions between DNA and the proteins that are in contact with it. One such reaction that can be envisioned is that the amino acids in these associated proteins may become crosslinked to the bases in DNA. Indeed such reactions do occur, and appear to be important processes that photoexcited DNA undergoes in vivo, as well as in DNA-protein complexes in vitro. [see below]
The first example of UV-induced crosslinking of DNA and proteins in a living system (Escherichia coli) was reported in 1962 (Smith, 1962). In the same study, it was noted that the treatment of E. coli with acridine orange and visible light also resulted in DNA-protein crosslinking. In fact, on the basis of the dose of radiation needed to produce the same reduction in colony formation, it was found that visible light plus dye crosslinked a larger percentage of the DNA than did UV radiation. On the other hand, X-irradiation produced little if any crosslinking (see below) (Smith, 1962). Since these early studies, photoinduced DNA-protein crosslinking has been observed in other cellular systems, as well as in isolated DNA-protein complexes. Among the latter are the crosslinking of histones to DNA in eukaryotic nucleosomes, the crosslinking of RNA polymerase and DNA polymerases to DNA, and the cross-linking of the gene 5 "melting" protein from fd phage to single-stranded DNA. (For reviews and references, see Smith, 1976; Shetlar, 1980; Saito and Sugiyama, 1990).
Detecting DNA-Protein Crosslinks
A number of methods have been developed for detecting DNA-protein cross-links (reviewed in Shetlar, 1980). The first method used was based upon the extraction of DNA free of protein from cells following UV irradiation. DNA can be isolated from E. coli free of protein using a detergent (sodium dodecyl sulfate) extraction procedure (Smith and Kaplan, 1961; Smith, 1962).
UV-Induced Crosslinks. With increasing doses of UV radiation there is a linear decrease in the amount of free DNA that can be extracted, and an increasing amount of DNA that remains associated with the denatured proteins (Figure 1).
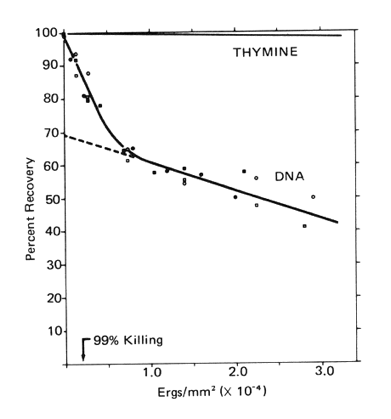
Figure 1. The extractability of DNA from E. coli B/r free of protein following increasing doses of UV radiation (254 nm). For comparison, the rate of loss of thymine due to the formation of thymine dimers is plotted.
[Modified from Smith, 1962]
Note that 30% of the DNA is seven times more sensitive to crosslinking with protein than is the remainder (see below). At a UV dose that kills 99% of the cells, about 10% of the DNA was crosslinked with protein (Smith, 1962).
As one might predict, it is the replicating portion of the DNA chromosome that is more sensitive to UV-induced crosslinking with protein (Smith, 1964a). This was tested by pulse labeling a log-phase culture of E. coli with tritiated thymine, and then replacing the radioactive thymine with non-radioactive thymine, and allowing the culture to continue growing exponentially. Samples were taken at various times over 2 generation times, irradiated and analyzed (Figure 2).
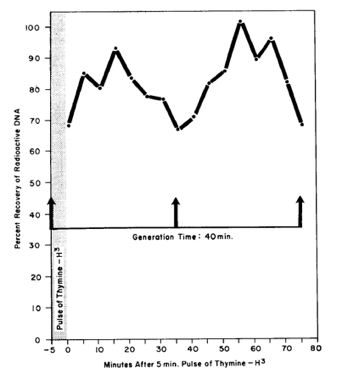
Figure 2. The sensitivity of a pulse labeled section of the bacterial DNA to be crosslinked to protein by a constant dose of UV radiation as a function of the time after the pulse. A log phase culture of E. coli 15TAU was pulsed with thymine-H3 for 5 min, the radioactive thymine was removed by filtration, the cells were returned to a medium containing non-radioactive thymine, and then allowed to continue logarithmic growth. At various times two aliquots were removed from the culture. One aliquot was UV irradiated with 133 ergs/mm2, and then both aliquots were treated with detergent for the isolation of DNA. The percent recovery of tritiated DNA free of protein versus the unirradiated control aliquots is plotted against the time following the pulse of thymine-H3. [Modified from Smith, 1964a]
This experiment suggests that the replication proteins are the ones most sensitive to DNA-protein crosslinking, i.e., the DNA-protein crosslinking was greatest immediately after the pulse labeling, and again one and two generations after the pulse labeling.
Photosensitized Crosslinks. When bacterial cells were pretreated with acridine orange or with methylene blue, and then exposed to intense visible light, survival deceased markedly, and a large amount of their DNA was crosslinked to protein. There is a correlation between the relative killing efficiency of the two dyes, and the relative production of DNA protein crosslinks (Figure 3).
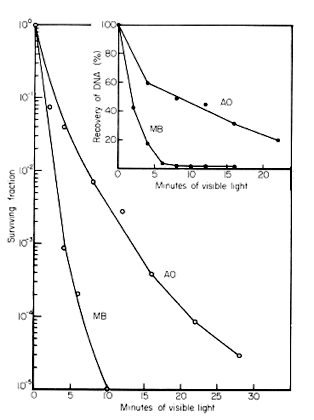
Figure 3. The killing of E. coli B/r, and the crosslinking of DNA and protein by visible light in the presence of acridine orange (AO) (3.4 µg/ml) or methylene blue (MB) (4 µg/ml). Stationary phase cells were washed and suspended in 0.1 M phosphate buffer (pH 6.8), and dye was added to the indicated concentrations. Solutions were irradiated with a 150 W GE floodlight (PAR-38) through 2.5 cm of water in a heavy Pyrex dish. Viability was determined on nutrient agar plates, and the DNA was isolated using the sodium dodecyl sulfate method (see above) (Smith and Kaplan, 1961; Smith, 1962). [Modified from Smith, 1976]
X-ray-Induced Crosslinks. After an X-ray does of 1 krad there was about a 5% loss in the extractability of DNA from E. coli B, but doses up to 40 krads did not alter this value (Smith, 1962). These results may be explained by the fact that X-rays produce a significant amount of single- and double-strand breaks in DNA, and the fact that the assay depends upon the selective precipitation of large molecular weight DNA crosslinked with protein.
X-ray-induced crosslinks are observed, however, if cells are irradiated under nitrogen (Barker et al., 2005). Under nitrogen, three times less DNA double-strand breaks are formed than when cells are X-irradiated under oxygen (Bonura et al., 1975). X-ray-induced DNA addition reactions are common, however, including DNA-DNA crosslinks (Myers, 1976).
Photoreactions of Nucleobases and Nucleosides with Amino Acids and Related Compounds
The first amino acid shown to photochemically add to uracil was cysteine, to form 5-S-cysteinyl-6-hydrouracil (Smith and Aplin, 1966). The chemical structure of the mixed photoproduct of thymine and cysteine was also determined (Smith, 1970) (Figure 4). Later work showed that this compound is photochemically-produced in two diastereomeric forms, and that two other compounds, namely 5-S-cysteinylmethyluracil (Varghese, 1973) and 5-S-cysteinyl-5,6-dihydrothymine (Shetlar and Hom, 1987) are also produced when thymine is irradiated in the presence of cysteine.
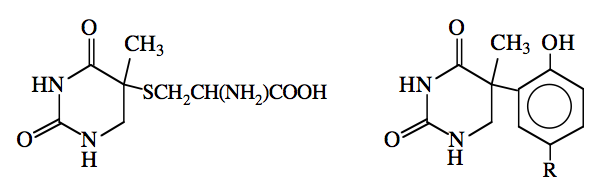
Figure 4. Adducts arising from the photoreactions of thymine with cysteine (left) and N-acetyltyrosine (right).
R = CH2CH(NH2)COOH
The photoreactivity of various polynucleotides for the addition of [35S]cysteine was also studied. Rate constants for this reaction were measured; poly rU was found to be the most reactive (k=21.8), followed by poly rC (k=8.1), poly dT (k=5.4), and poly rA (k=0.6). Ribonucleic acid showed a biphasic response with k=21.8 and k=4.8 (Smith and Meun, 1968).
In addition to the products of the photoreactions of thymine and uracil with cysteine, photoproducts have been characterized in a number of other systems containing nucleobases (or nucleosides), and amino acids (or amino acid analogs) (reviewed in Saito and Sugiyama, 1990). For example, the reaction of thymine with the phenolic ring system contained in N-acetyltyrosine results in a compound with the structure shown in Figure 4 (Shaw et al., 1998), while the reaction of thymidine with lysine yields a photoproduct of a very different nature (Figure 5) (Saito et al., 1981, 1983a). The production of this compound involves the attack of the ε-amino moiety of the lysine on the carbonyl group in the 2-position of thymidine. There is evidence that cytosine and 5-methylcytosine (a minor nucleobase found in eukaryotic DNA) photoreact with lysine to form adducts of a similar nature (Dorwin et al., 1988).
Interestingly, 5-methylcytosine also reacts with cysteine analogs (e.g., 3-mercaptopropionic acid) to form a 5-methylcytosine product with a structure analogous to that of the lysine adduct shown in Figure 5 (i.e., with NHR2 being replaced with a SR2 moiety and R1 = H) (Shetlar and Chung, 2013).
Other recent work (Shetlar et al., 2013) indicates that 2’-deoxycytidine photohydrates (as well as 2’-deoxyuridine and uridine hydrates) react with alkylamines (and polyamines) in a secondary dark reaction at near neutral pH to form a deoxycytidine adduct (deoxyuridine adduct, uridine adduct), analogous to that shown in Figure 5, in which the adducted amine moiety becomes attached as NR1; the sugar moiety is attached as NHR2. Early work (Janion and Shugar, 1967) showed that dihydrocytosines can undergo transamination reactions in the dark, in which aliphatic amines displace the 4-amino group of the dihydro compound to form "transaminated" dihydrocytosines. This same type of reaction also occurs with cytosine nucleoside photohydrates, in which the 5,6 bond is saturated. In particular, it has been shown that this reaction occurs when the RNA bacteriophage MS2 is irradiated, leading to crosslinking of lysine residues in coat protein to the genomic nucleic acid (Budowsky et al., 1976). These results suggest that secondary dark reactions, subsequent to the formation of primary photoproducts in DNA, may play a role in DNA-protein crosslinking. Determination of the contributions of various secondary crosslinking reactions to the total photochemistry of DNA in its cellular environment, may be a fertile field for further study.
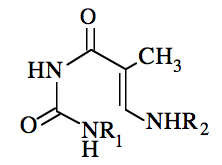
Figure 5. Structure of the photoproduct arising from the reaction of thymidine with lysine. R1 is (CH2)4CH(NH2)COOH, and R2 is a 2'-deoxyribosyl moiety.
Other amino acids are also reactive with nucleobases and polynucleotides. The first survey performed determined the ability of the 22 common amino acids to add photochemically (254 nm) to uracil. The 11 reactive amino acids were glycine, serine, phenylalanine, tyrosine, tryptophan, cystine, cysteine, methionine, histidine, arginine and lysine. The most reactive amino acids were phenylalanine, tyrosine and cysteine. Therefore, the photochemical addition of amino acids to uracil appears to be a fairly common phenomenon (Smith, 1969). A later study of the photoreactivity of polyuridylic acid indicated that all 20 amino acids were reactive, as well as a variety of glycylpeptides and other peptides (Shetlar et al., 1984c).
When thymine was similarly screened for photochemical reactivity with 22 common amino acids, only lysine, arginine, cysteine and cystine formed heteroadducts after exposure times similar to those used for uracil. Thymine was generally less reactive than uracil with amino acids when exposed to UV radiation (Schott and Shetlar, 1974). This may be due to the by shielding of carbon-5 in thymine by the methyl group at that position.
Another study used a fluorescence assay method to assess the photoreactivity of DNA and polynucleotides for the addition of various amino acids and peptides. The reactivities of the 20 amino acids commonly occurring in proteins were determined for their photochemical addition to denatured calf thymus DNA at pH 7. Fifteen amino acids were reactive, with cysteine, lysine, phenylalanine, tryptophan, and tyrosine being the most reactive. Alanine, aspartic acid, glutamic acid, serine, and threonine were unreactive (Shetlar et al., 1984a). Corresponding quantum yields were also determined for many of the glycyldipeptides (e.g., glycylserine) of the same amino acids. It was found that of the peptides studied, those containing lysine, cystine, proline, histidine and the various aromatic acids (phenylalanine, tyrosine, tryptophan) were the most reactive (glycylcysteine was not studied). Interestingly, in peptide form, all of the amino acids studied displayed some degree of reactivity. In almost all cases, amino acids incorporated into peptides had higher reactivities towards photoaddition than the corresponding carboxyl terminal amino acids at the same concentration.
Measurements similar to those done on DNA photoreactivity were made on the photochemical reactivity of four polyribonucleotides, namely poly rA, poly rC, poly rG, and poly rT, towards the addition of glycine and the L-amino acids commonly occurring in proteins, excluding proline (Shetlar et al., 1984b). Poly rA was reactive with eleven of the twenty amino acids tested, with phenylalanine, tyrosine, glutamine, lysine and asparagine being the most reactive. Poly rG reacted with sixteen amino acids; phenylalanine, arginine, cysteine, tyrosine, and lysine displayed the largest quantum yields. Poly rC showed photoreactivity with fifteen amino acids, with phenylalanine, lysine, cysteine, tyrosine and arginine having the highest reactivities. Poly rT was reactive with fifteen of nineteen amino acids surveyed, and showed the highest quantum yields for cysteine, phenylalanine, tyrosine, lysine and asparagine. None of the polynucleotides were reactive with aspartic acid or glutamic acid. Studies on the photoaddition of various glycyldipeptides with each of the polynucleotides indicated that they were often more reactive than the amino acids themselves. In general, poly rT was the most reactive polynucleotide towards photoaddition for most of the amino acids and peptides studied. For example, the quantum yields for the photoaddition of phenylalanine to poly rT, poly rC, poly rA, and poly rG were in the ratio of 80:4:5:3.
Bromo- and iodo-substituted uracils and cytosines are also capable of photoreaction with amino acids. For example, 5-bromouracil photocouples with tyrosine, tryptophan and histidine (Dietz and Koch, 1987), as well as peptide linkages (Dietz et al., 1987). 5-Bromouracil also reacts with ethylamine (Shetlar et al., 1991), a lysine analog, to form a compound analogous to that formed in the reaction of thymine with lysine (Figure 5).
Cells of E. coli whose thymine has been replaced by 5-bromouracil are more sensitive to killing by UV irradiation than are unsubsititued cells (Greer, 1960; Kaplan et al., 1962), and they show a 5-fold greater sensitivity of UV-induced DNA-protein crosslinking than do unsubstituited cells (Smith, 1964b).
Photoinduced Crosslinks in DNA-Protein and Related Systems
A number of nucleobase-amino acid crosslinks have been identified in various UV-irradiated DNA-protein and related systems. For example, thymine-lysine conjugates have been identified as participants in the crosslinking in DNA-histone systems (Saito et al., 1983b; Kurochkina et al., 1987). Thymine-cysteine conjugates have been shown to be produced in the UV-induced crosslinking of the gene 5 protein of fd phage to its corresponding DNA (Paradiso and Konigsberg, 1982). At the level of a nucleoside-peptide system, the single tyrosine contained in Angiotensin I was crosslinked to thymidine when a solution containing these two components was irradiated (Shaw et al., 1992). Similar results were obtained when thymidine, thymidine-5'-phosphate and thymidylyl-[3'-5']-2'-deoxyadenosine were irradiated in the presence of the tyrosine-containing heptad repeat peptide unit found in the largest subunit of the eukaryotic RNA polymerase II multiprotein complex (Connor et al., 1998a).
Photocrosslinking as a Tool for Structural Studies of DNA-Protein Complexes
DNA-protein crosslinking is a valuable tool for studying the structure of DNA-protein complexes. Since the only amino acids and nucleobases that can participate in crosslinking are those in contact in DNA-protein complexes, crosslinking can potentially be used to identify amino acids (or peptides) and nucleobases in those regions involved in binding protein to DNA. While steady state UV irradiation has been used in many studies, the use of pulsed lasers, in conjunction with mass spectrometry, has provided a powerful alternative approach to studying crosslinking, especially for examining contacts in native DNA-protein complexes. For example, this combination of techniques has been used to identify six crosslinked peptides in the complex formed between the single-stranded DNA binding domain of rat DNA polymerase ß, and the oligonucleotide d(ATATATA) (Connor et al., 1998b). [Experimental aspects of the use of crosslinking to study the structure of nucleic acid-protein complexes are discussed by Williams and Konigsberg (1992) (by steady state UV-induced crosslinking), and by Hockensmith et al. (1992) (by laser pulse-induced crosslinking). Reviews by Meisenheimer and Koch (1997), and Steen and Jensen (2002), provide information about, and references to, a number of studies in which the UV-induced crosslinking of nucleic acid-protein complexes has been used to gain structural information about such complexes.]
DNA-protein complexes in which the DNA component has been modified to contain 5-halogenated uracils or cytosines have also been studied using laser crosslinking-mass spectrometric approaches. For example, it has been shown that tryptophans 54 and 88 in the sequence of the E. coli single-stranded DNA binding protein can be bound to a DNA oligomer in which thymines are replaced with 5-iodouracil moieties (Steen et al., 2001). Nucleic acid-protein complexes, in which thymine has been replaced with 5-bromouracil or cytosine with 5-iodocytosine, have also found use in photochemical experiments designed to study contact regions in these complexes. [For reviews, see Meisenheimer and Koch, 1997; Steen and Jensen, 2002]
Biological Importance and Repair of DNA-Protein Crosslinks
Since the crosslinking of DNA and protein by UV radiation is many times more sensitive analytically than is thymine dimer formation, it was suggested that DNA-protein crosslinks may play a significant role in the inactivation of bacteria by UV radiation (Smith, 1962).
This hypothesis was subsequently proven by growing E. coli mutants under different conditions that affect cell sensitivity to UV radiation. A direct correlation was observed between the amount of DNA crosslinked to protein by a given dose of UV radiation, and the intrinsic sensitivity to killing by UV radiation under the several growth conditions studied (Smith et al., 1966).
In addition, the increased sensitivity of E. coli to killing by UV irradiation when frozen, and the variation in this sensitivity as a function of the temperature during irradiation, correlated with changes in the amount of DNA that was crosslinked to protein by UV irradiation. These variations in sensitivity to killing did not correlate with the production of thymine dimers (Smith and O'Leary, 1967).
These results on the biological importance of UV radiation-induced DNA-protein crosslinks are consistent with the fact that only about 60% of the survival of E. coli after UV irradiation can be photoreactivated (see module on Phototreactivation), i.e., 40% of lethality must be due to lesions other than cyclobutane pyrimidine dimers. DNA-protein crosslinks cannot be photoreactivated (Smith, 1964b).
DNA-protein crosslinks are repaired by postreplication repair, and they cause a longer delay in DNA synthesis than do pyrimidine dimers (Smith and Hamelin, 1977). [See module on Recombinational DNA Repair]
It should be noted that the formation of DNA-protein crosslinks in cellular systems is induced by stresses other than the absorption of radiation (e.g., chemically-induced reactions and crosslinking mediated by reactive oxygen species). Descriptions of relevant early work on other types of nucleic acid-protein crosslinks is given in Smith (1976), while a more recent review of the formation of some of these types of lesions, as well as their repair and biological consequences, is provided by Barker et al. (2005).
Summary and Conclusions
DNA-protein crosslinks are important lethal lesions in cells exposed to UV radiation. Crosslinks are particularly disruptive, as they occur mostly in the area of the chromosome that is undergoing replication. The structures of a number of adducts that are potentially responsible for crosslinks formed in UV-irradiated DNA-protein complexes have been determined. Surveys of the reactivity of thymine and uracil, as well as polynucleotides of the DNA and RNA nucleobases towards the photoaddition of amino acids have been conducted. Photocrosslinking is a useful tool to map DNA-protein contacts in DNA-protein complexes.
References
Barker, S, Weinfeld, M, Murray, D. (2005) DNA–protein crosslinks: their induction, repair, and biological consequences. Mut Res 589: 111-135.
Bonura, T, Town, CD, Smith, KC, Kaplan, HS. (1975) The influence of oxygen on the yield of DNA double-strand breaks in X-irradiated Escherichia coli K-12. Rad Res 63: 567-577.
Budowsky, EI, Simukova, NA, Turchinsky, MF, Boni, IV, Skoblov, YM. 1976. Induced formation of covalent bonds between nucleoprotein components. V. UV or bisulfite induced polynucleotide-protein crosslinkage in bacteriophage MS2. Nucleic Acids Res: 3: 261-276.
Connor, DA, Falick, AM, Shetlar, MD. 1998a. UV light-induced cross-linking of nucleosides, nucleotides and a dinucleotide to the carboxy-terminal heptad repeat peptide of RNA Polymerase II as studied by mass spectrometry. Photochem. Photobiol. 68: 1-8.
Connor, DA, Falick, AM, Young, MC, Shetlar, MD. 1998b. Probing the binding region of the single-stranded DNA-binding domain of rat DNA polymerase using nanosecond-pulse laser-induced cross-linking and mass spectrometry. Photochem. Photobiol. 68: 299-308.
Dietz, TM, Koch, TH. 1987. Photochemical coupling of 5-bromouracil to tryptophan, tyrosine and histidine, peptide like derivatives in aqueous fluid solution. Photochem. Photobiol. 46: 971-978
Dietz, TM, von Trebra, RJ, Swanson, BJ, Koch, TH. 1987. Photochemical coupling of 5-bromouracil (BU) to a peptide linkage. A model for BU-DNA photocrosslinking. J. Amer. Chem. Soc. 109: 1793-1797
Dorwin, EL, Shaw AA, Hom K, Bethel P, Shetlar, MD. 1988. Photoexchange products of cytosine and 5-methylcytosine with
N-
 -acetyl-L-lysine and L-lysine. J. Photochem. Photobiol. B. 2: 265-278.
-acetyl-L-lysine and L-lysine. J. Photochem. Photobiol. B. 2: 265-278.
Greer, S. 1960. Studies on ultraviolet irradiation of Escherichia coli containing 5-bromouracil in its DNA. J. Gen. Microbiol. 22:618-634.
Hockensmith, JW, Kubasek, WL, Vorachek, WR, Evertsz, EM, von Hippel, PH. 1991. Laser cross-linking of protein-nucleic acid complexes. Methods Enzymol. 208, 211-236.
Janion, C. and Shugar D. 1967. Reaction of amines with dihydrocytosine analogs and formation of aminoacid and peptidyl dervivatives of diydropyrimidines. Acta Biochim. Pol. 14: 293-302.
Kaplan, HS, Smith, KC, Tomlon, PA. 1962. Effect of halogenated pyrimidines on radiosensitivity of E. coli. Rad. Research, 16:98-113.
Kurochkina, LP, Komissarov, AA, Kolomiitseva, GY. 1987. Localization of the lysine residue in histone H3 forming a thymine-lysine cross-link when deoxyribonucleoprotein is irradiated with UV light. Biochem. USSR, 52: 1457-1461.
Meisenheimer, KM, Koch, TH. 1997. Photocross-linking of nucleic acids to associated proteins. Crit. Rev. Biochem. Mol. Biol. 32: 101-140.
Myers, LS, Jr. 1976. Ionizing radiation-induced attachment reactions of nucleic acids and their components, in Aging, Carcinogenesis, and Radiation Biology (the role of nucleic acid addition reactions), (KC Smith, ed.), Plenum Press, New York, pp. 261-286.
Paradiso, PR, Konigsberg, W.,1982. Photochemical cross-linking of the gene-5 protein fd DNA complex from fd-infected cells. J. Biol. Chem. 257: 1462-1467 (and references therein).
Saito, I, Sugiyama, H, Ito, S, Furukawa, N, Matsuura, T., 1981. A novel photoreaction of thymidine with lysine. Photoinduced migration of thymine from DNA to lysine. J. Amer. Chem. Soc. 103:1598-1600.
Saito, I, Sugiyama, H, Matsuura, T. 1983a. Photoreaction of thymidine with alkylamines. Application to selective removal of thymine from DNA. J. Amer. Chem. Soc. 105: 956-962
Saito, I, Sugiyama H, Matsuura, T. 1983b. Isolation and characterization of a thymine-lysine adduct in UV-irradiated nuclei. The role of thymine-lysine photoaddition in photo-cross-linking of proteins to DNA. J. Amer. Chem. Soc. 105: 6989-6991
Saito, I, Sugiyama, H. 1990. Photoreactions of nucleic acids and their constituents with amino acids and related compounds, in Biooorganic Photochemistry (H. Morrison, ed), Vol. 1, John Wiley and Sons, New York, pp 317-340.
Schott, HN, Shetlar, MD. 1974. Photochemical addition of amino acids to thymine. Biochem. Biophys. Res. Comm. 59:1112-1116.
Shaw, AA , Falick, AM, Shetlar, MD. 1992. Photoreactions of thymine and thymidine with N-
 -acetyltyrosine. Biochemistry 31: 10976-10983
-acetyltyrosine. Biochemistry 31: 10976-10983
Shetlar, MD. 1980. Cross-linking of proteins to nucleic acids by ultraviolet light. Photochem. Photobiol Rev. 5: 105-197.
Shetlar, MD, Christensen, J, Hom, K. 1984a. Photochemical addition of amino acids and peptides to DNA. Photochem. Photobiol. 39:125-133.
Shetlar, MD, Hom, K, Carbone, J, Moy, D, Steady, E, Watanabe, M. 1984b. Photochemical addition of amino acids and peptides to homopolyribonucleotides of the major bases. Photochem. Photobiol. 39: 135-140.
Shetlar, MD, Carbone, J, Steady E, and Hom K. 1984c. Photochemical addition of amino acids and peptides to polyuridylic acid. Photochem. Photobiol. 39: 141-144
Shetlar, MD, Hom, K. 1987. Mixed products of thymine and cysteine produced by direct and acetone sensitized photoreactions. Photochem. Photobiol. 45: 703-712.
Shetlar, MD, Rose, RB, Hom, K, Shaw, AA. 1991. Ring opening photoreactions of 5-bromouracil and 5-bromo-2'-deoxyuridine with selected alkylamines. Photochem. Photobiol. 53: 595-609.
Shetlar, MD, Chung, J. 2013. Ring-opening photoreactions of 5-methylcytosine with 3-mercaptopropionic acid and other thiols. Photochem. Photobiol. 89: 878-883.
Shetlar, MD, Hom, K., Venditto, VJ. 2013. Photohydrate-mediated reactions of uridine, 2'-deoxyuridine and 2'-deoxycytidine with amines at near neutral pH. Photochem. Photobiol. 89, 868-877.
Smith, KC. 1962. Dose dependent decrease in extractability of DNA from bacteria following irradiation with ultraviolet light or with visible light plus dye. Biochem. Biophys. Res. Commun., 8:157-163.
Smith, KC. 1964a. The photochemical interaction of deoxyribonucleic acid and protein in vivo and its biological importance. Photochem. Photobiol., 3:415-427.
Smith, KC. 1964b. Photochemistry of the Nucleic Acids, in Photophysiology (A.C. Giese, ed), Academic Press, New York, Vol. 2, pp. 329-388.
Smith, KC. 1969. Photochemical addition of amino acids to 14C-uracil. K.C. Smith, Biochem. Biophys. Res. Commun., 34:354-357.
Smith, KC. 1970. A mixed photoproduct of thymine and cysteine: 5-S-cysteine, 6-hydrothymine. Biochem. Biophys. Res. Commun., 39:1011-1016.
Smith, KC. 1976. Radiation-induced crosslinking of DNA and protein in bacteria, in Aging, Carcinogenesis, and Radiation Biology (the role of nucleic acid addition reactions), (KC Smith, ed.), Plenum Press, New York, pp. 67-81.
Smith, KC, Aplin, RT. 1966. A mixed photoproduct of uracil and cysteine (5-S-cysteine-6-hydrouracil). A possible model for the in vivo crosslinking of deoxyribonucleic acid and protein by ultraviolet light. Biochemistry 5:2125-2130.
Smith, KC, Hamelin, C. 1977. DNA synthesis kinetics, cell division delay, and post-repliction repair after UV irradiation of frozen cells of E. coli B/r. Photochem. Photobiol. 25:27-29.
Smith, KC, Hodgkins, B, O'Leary, ME. 1966. The biological importance of ultraviolet light induced DNA-protein crosslinks in Escherichia coli 15 TAU. Biochim. Biophys. Acta 114:1-15.
Smith, KC, Kaplan, HS. 1961. A chromatographic comparison of the nucleic acids from isologous newborn, adult, and neoplastic thymus. Cancer Res. 21:1148-1153.
Smith, KC, Meun, DHC. 1968. Kinetics of the photochemical addition of [35S]cysteine to polynucleotides and nucleic acids. Biochemistry 7:1033-1037.
Smith, KC, O'Leary, ME. 1967. Photoinduced DNA-protein cross-links and bacterial killing: A correlation at low-temperatures. Science 155: 1024-1026.
Steen, H, Jensen, ON. 2002, Analysis of protein-nucleic acid interactions by photochemical cross-linking and mass spectrometry. Mass Spectrometry Reviews 21: 163-182
Steen, H., Peterson, J., Mann, M. , Jensen, ON. 2001. Mass spectrometric analysis of a UV-cross-linked protein-DNA complex: Tryptophans 54 and 88 of E. coli SSB cross-link to DNA. Protein Sci.10: 1989-2001
Varghese, AJ. 1973. Properties of photoaddition products of thymine and cysteine. Biochemistry 12: 2725-2730.
Williams, KR, Konigsberg, WH. 1991. Identification of amino acid residues at interface of protein-nucleic acid complexes by photochemical crosslinking. Methods Enzymol. 208, 516-539.
10/11/08
06/02/11
11/24/13
04/09/14